-
PDF
- Split View
-
Views
-
Cite
Cite
Jun Zhou, Aizhen Sun, Da Xing, Modulation of cellular redox status by thiamine-activated NADPH oxidase confers Arabidopsis resistance to Sclerotinia sclerotiorum, Journal of Experimental Botany, Volume 64, Issue 11, August 2013, Pages 3261–3272, https://doi.org/10.1093/jxb/ert166
Close - Share Icon Share
Abstract
Sclerotinia sclerotiorum can initially suppress host oxidative burst to aid infection establishment, but later promotes reactive oxygen species (ROS) generation as proliferation advances. Here, it was shown that the cellular redox status can be modulated by thiamine to protect Arabidopsis thaliana against Sclerotinia at the early stages of infection. The initial inhibition of host ROS generation by Sclerotinia-secreted oxalate could effectively be alleviated by thiamine. Thiamine pre-treatment and subsequent wild-type Sclerotinia invasion induced an increase of ascorbate peroxidase activity concomitant with decreased ascorbate/dehydroascorbate ratios, which led to the cellular transition towards oxidative status in infected tissues. Particularly, it was observed that wild-type Sclerotinia, but not oxalate-deficient A2 mutant, could suppress the activity of NADPH oxidase (NOX), which might be an important mechanism underlying the early inhibition of ROS burst. Nevertheless, thiamine pre-treatment followed by wild-type Sclerotinia infection promoted NOX-derived ROS accumulation. Further studies showed that cytosolic Ca2+ and staurosporine-sensitive protein kinase(s) participated in thiamine-induced activation of NOX. Moreover, thiamine-induced tissue defence responses including callose/lignin deposition and stomatal closure were closely correlated with NOX-derived ROS generation. Additionally, studies with Brassica species indicated that the regulation of thiamine is largely conserved upon Sclerotinia infection. Collectively, it was concluded that thiamine reverses the initial reducing status through activating NOX-dependent ROS signalling to perturb the disease progress of Sclerotinia.
Introduction
Sclerotinia sclerotiorum, known as white mould, is a necrotrophic fungal pathogen with a broad host range (Purdy, 1979). Early in pathogenesis, Sclerotinia secretes up to 10mM oxalate as host colonization advances (Maxwell and Lumsden, 1970). Oxalate, a simple but versatile molecule, can commandeer programmed cell death (PCD) in plants (Errakhi et al., 2008; Kim et al., 2008), destroy pectin structure by extracting calcium ions (Ca2+) (Bateman and Beer, 1965), and inhibit abscisic acid (ABA)-induced stomatal closure (Guimarães and Stotz, 2004). In addition, Sclerotinia-secreted oxalate can inhibit the host’s reactive oxygen species (ROS) burst and the consequent defence responses (Cessna et al., 2000). However, reduction of oxalate with a deficient mutant or overexpression of oxalate oxidase leads to ROS generation, which enables the host plant to tune resistance responses (Cessna et al., 2000; Hu et al., 2003; Dong et al., 2008). Interestingly, Williams et al. (2011) also demonstrated that oxalate-induced inhibition of ROS generation only occurs at the initial stages of infection to aid pathogen compatibility, with promotion of ROS production at the later stages as fungal proliferation advances. It is well known that early recognition of an intruder is critical to halt its invasion. Thus, ROS generation appears to be an important determinant during initial recognition of Sclerotinia invasion in plants (Perchepied et al., 2010). However, the mechanism underlying the early inhibition of the ROS burst is unknown.
Among various ROS-generating enzymatic systems in plant, NADPH oxidases (NOX), also known as respiratory burst oxidase homologues (Rbohs), are the most thoroughly studied (Marino et al., 2012). Rboh mutants are more susceptible than the wild-type Arabidopsis to Sclerotinia infection (Perchepied et al., 2010). Diphenyleneiodonium (DPI), an inhibitor of NOX, has been shown to interfere with oxalate-induced plant PCD (Kim et al., 2008). Moreover, ROS production in response to oxalate-deficient Sclerotinia is strongly reduced in AtrbohD plants, but not in AtrbohF mutants (Guo and Stotz, 2010; Perchepied et al., 2010). NOX-mediated ROS signalling may be of prominent significance in plant defence against Sclerotinia infection.
The interaction between ROS and antioxidants is crucial for maintaining cellular redox homeostasis, which is a key factor in determining the outcome following pathogen challenge in plants (Foyer and Noctor, 2005, 2011; Sun et al., 2012). Ascorbate and glutathione, as multifunctional metabolites, are important in redox homeostasis and signalling in the plant response to pathogens (Pastori et al., 2003; Parisy et al., 2007). To achieve pathogenic success, the host cell redox homeostasis is perturbed by Sclerotinia-secreted oxalate (Williams et al., 2011). However, during the process of infection establishment, the role of ascorbate and glutathione in oxalate-manipulated cell redox status has yet to be determined.
Sclerotinia can readily infect its host and spread rapidly under favourable conditions, which makes it difficult to control the disease (Purdy, 1979). The inducible basal resistance of the plant to the already colonizing pathogen is often too weak and too late to prevent disease. However, the priming induced by some plant activators (e.g. low doses of salicylic acid, β-aminobutyric acid, thiamine) are capable of inducing rapid and effective defence responses to halt the invading pathogen (Thulke and Conrath, 1998; Ton and Mauch-Mani, 2004; Ahn et al., 2005). Thiamine pyrophosphate (TPP) is well known for its role as a co-factor of enzymes involved in the tricarboxylic acid cycle, the pentose phosphate pathway, branched chain amino acid biosynthesis, and isoprenoid biosynthesis (Goyer, 2010). In addition, thiamine also enables plants to counteract various abiotic stresses like salt, heat, and oxidative stress (Rapala-Kozik et al., 2008, 2012; Tunc-Ozdemir et al., 2009). While in vitro studies show that thiamine has an antioxidative activity (Jung and Kim, 2003), it is still unclear whether the protective effects of thiamine result from its direct antioxidant effect (Asensi-Fabado and Munné-Bosch, 2010). Furthermore, Ahn et al. (2007) reported that the defence signalling induced by thiamine is dependent on hydrogen peroxide (H2O2) and removal of H2O2 nullifies plant defence responses. It is noteworthy that thiamine apparently influences several signal transduction pathways that are linked to plant defence, including fluxion of Ca2+ and upregulation of protein kinase C-like activity (Ahn et al., 2005), which are the master regulators of NOX during stress responses (Sagi and Fluhr, 2001; Jiang et al., 2011). Nevertheless, whether thiamine can activate NOX involved in cellular defence signalling, especially in plant resistance to Sclerotinia, remains unclear.
This study aimed to address whether thiamine regulates the signalling pathways of Arabidopsis resistance to the necrotrophic pathogen Sclerotinia. Our results revealed that thiamine could reverse the initial reducing status through activating NOX-mediated ROS signalling to perturb the disease progress of Sclerotinia. The findings contribute to our understanding of plant–Sclerotinia interactions, as well as to control of Sclerotinia in practice.
Materials and methods
Plant material and chemicals treatment
Arabidopsis ecotype Columbia-0 (Col-0) and mutant seeds tz-1 (N3375), AtrbohD (N9555) and AtrbohDF (N9558) were obtained from the European Arabidopsis Stock Centre. For Brassica napus, experiments were performed using two cultivars of the susceptible genotype ‘Yinong 34’ and the partially resistant ‘Zhongshuang 9’ (Wang et al., 2004). Plants were cultivated in growth cabinets at 22 °C (day) and 18 °C (night), with a 16h light period (300 μmol m–2 s–1). Thiamine, DPI, BAPTA-AM, EGTA, staurosporine, sodium, 3ʹ-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) bezene sulfonic acid hydrate (XTT) were purchased from Sigma-Aldrich. Thiamine treatment was according to Ahn et al. (2005). Leaves from 4-week-old plants were sprayed with 250 μg ml–1 of Tween 80 (control) or thiamine (dissolved in 250 μg ml–1 Tween 80) at 4h prior to Sclerotinia inoculation. For DPI treatment, leaves were inoculated with Sclerotinia at 30min after pre-infiltrated with 100 μM DPI.
Fungal growth and inoculations
A wild-type isolate (1980) and an oxalate-deficient mutant (A2) of Sclerotinia were grown on potato-dextrose agar at 21 °C for 3 d. Agar plugs (diameter 0.3 or 0.8cm) containing the growing mycelia were used to inoculate the leaves. Infected plants were kept in a clear plastic box under saturating humidity. Oxalate levels were determined following the method of Huang et al. (2011). Photographs of the necrotic phenotype were produced using a SONY numeric camera (HDR-XR500E). For lesion size measurement, photographs were captured using a Zeiss inverted microscope installed with a Carl Zeiss AxioCam MRc5 camera. Lesion area was quantified at least in ten leaves with the measurement tool ‘outline spline’ in AxioVision Rel.4.5 software.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was extracted from Arabidopsis leaves using Trizol according to the supplier’s recommendations. First-strand cDNA was synthesized using the SuperScript II First-Strand Synthesis System (Invitrogen). qRT-PCR was performed using the Roche LightCyclerTM 2.0 Real-time PCR Detection System. The expression of target gene was normalized relative to the housekeeping gene ACTIN2 (Sun et al., 2012). The RBOHD, RBOHF, THI1, TH1, and TPK primer sequences used have been described elsewhere (Penfield et al., 2006; Rapala-Kozik et al., 2012).
Measurement of thiamine and its phosphate analogues
Thiamine and its phosphate analogues were converted into thiochrome by adding cyanogen bromide, and were measured according to the method of Rapala-Kozik et al. (2008, 2012). Samples were quantified by reverse-phase high-pressure liquid chromatography (RP-HPLC). An Agilent ZORBAX Eclipse plus C18 (5 μm) column (150×4.6mm) was used for the separation.
Measurement of superoxide anion (O2–) and H2O2
O2– was measured according to Jiang and Zhang (2002) by analysis of XTT formazan absorbance at 470nm. Corrections were made for the background absorbance in the presence of superoxide dismutase (SOD; 50U). The accumulation of O2– was also monitored in situ by nitroblue tetrazolium (NBT) as described previously (Jabs et al., 1996). Microscopic images were photographed with an Olympus BX51 microscope. For quantitative determination of H2O2, an Amplex Red Hydrogen Peroxide Assay kit (Invitrogen) was used.
NOX activity determination
Plasma membranes were isolated as described by Liu et al. (2012). For native PAGE, protein samples from each fraction (20 μg per lane) were separated in a 7.5% (w/v) polyacrylamide separating gel and 4% (w/v) stacking gels at 4 °C. The gel was incubated in the dark with NBT solution [50mM Tris-HCl (pH 7.4), 0.2mM NBT, 0.1mM MgCl2 and 1mM CaCl2] for 20min. The reaction was initiated by addition of 0.5mM NADPH and stopped when clear blue formazan bands were observed.
The NOX activity of plasma membrane vesicles was also determined spectrophotometrically by the reduction of XTT (Sagi and Fluhr, 2001). The analysis reaction medium contained 10 μg protein, 0.3mM XTT, and 0.18mM NADPH in 1ml 50mM Tris-HCl buffer (pH 7.4). The activity was expressed as the difference in XTT formazan absorbance at 470nm (∆A470) in the presence or absence of 50U of SOD.
Enzyme assays, and ascorbate and glutathione determination
The activities of the antioxidant enzymes SOD, ascorbate peroxidase (APX), and glutathione reductase (GR) were measured spectrophotometrically as described previously (Rao et al., 1996). Ascorbate was assayed according to the method of Queval and Noctor (2007) based on the change in absorbance of APX at 265nm. The levels of glutathione were determined with a reduced glutathione and oxidized glutathione assay kit (Beyotime).
Measurement of cytosolic calcium concentration ([Ca2+]cyt)
The method for [Ca2+]cyt detection was based on previous work (Yue et al., 2012). Ca2+ was stained with Fluo-3-AM ester (Invitrogen), which is hydrolysed to yield genuine Fluo-3 capable of indicating changes in [Ca2+]cyt. The fluorescence intensity of Fluo-3 was measured with a fluorescence spectrometer (excitation at 488nm, emission at 525nm).
Cell wall fortification and stomatal aperture measurements
Callose deposition was stained with 0.01% (w/v) aniline-blue and observed using a fluorescence microscope (Sun et al., 2012). Lignin was stained with phloroglucinol as described by Vallet et al. (1996), and the content was measured by determining absorbance at 280nm with a thioglycolic acid assay. Stomatal aperture was monitored as described by Guimarães and Stotz (2004). Epidermal peels were prepared from the areas surrounding necroses, and incubated in the dark for at least 3h to induce stomatal closing. The stomatal aperture was quantified with AxioVision Rel.4.5 software and is given as width/length.
Results
Thiamine protects Arabidopsis against Sclerotinia invasion
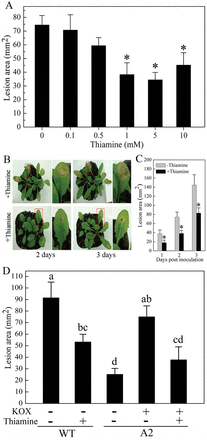
Thiamine has been determined to confer plant resistance to a wide range of pathogens (Ahn et al., 2005), which impelled us to explore whether thiamine was involved in Arabidopsis defence against Sclerotinia. Thiamine showed a concentration-dependent resistance effect on necrotic lesions, which were reduced by 52% following treatment with 1mM thiamine relative to untreated plants (Fig. 1A). Since no further increase of resistance effect was observed at higher concentrations, thiamine at 1mM was used in the following experiments unless otherwise mentioned. Furthermore, pre-treatment of thiamine significantly delayed progression of lesion expansion (Fig. 1B, C), but did not directly affect the fungal hyphae growth in vitro (Supplementary Fig. S1A, Supplementary Data at JXB online).
Thiamine enhances Arabidopsis resistance to Sclerotinia. (A) Lesion development on Sclerotinia-infected leaves pre-treated with 0–10mM thiamine. Lesion areas were measured at 2 d. (B, C) Disease symptoms (B) and lesion area (C) on Sclerotinia-inoculated leaves pre-treated with 1mM thiamine. (D) Thiamine reduces the recovery of pathogenicity of A2 mutant. Leaves were pre-infiltrated with 10mM potassium oxalate (KOX) (pH 7.0) to recover the pathogenicity of the A2 mutant. Values represent means ±standard deviation (SD) of at least ten lesions. Asterisks indicate significant differences from thiamine-untreated samples (Student’s t-test, P <0.05). Different letters indicate statistically significant differences (Duncan’s multiple range tests; P <0.05). (This figure is available in colour in JXB online.)
Since oxalate is responsible for the pathogenicity of Sclerotinia (Godoy et al., 1990; Cessna et al., 2000), we evaluated necrotic lesions with an oxalate-deficient Sclerotinia mutant (A2) in leaves pre-treated with thiamine. Oxalate accumulation in leaves infected with A2 mutant was much less than that with wild-type Sclerotinia (Supplementary Fig. S1C). Compared with wild-type Sclerotinia, A2 mutant invasion resulted in restricted necrotic lesions, and 10mM potassium oxalate (pH 7.0) remarkably restored A2 pathogenicity. However, the recovery of A2 pathogenicity could also be significantly contracted by exogenous application of thiamine (Supplementary Data). Together, these results illustrated that thiamine could induce Arabidopsis resistance to Sclerotinia by alleviating oxalate-induced necrosis.
The impact of endogenous thiamine on defence against Sclerotinia infection
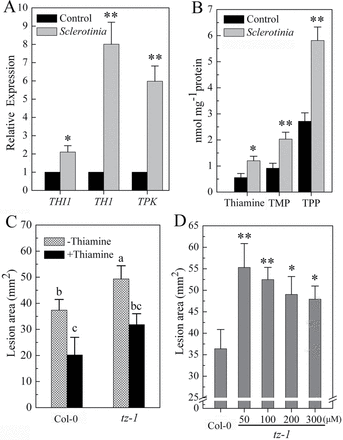
To assess whether the endogenous thiamine biosynthesis was influenced by Sclerotinia infection, we analysed the expression of genes responsible for the biosynthesis of the thiazole moieties of thiamine (THI1) and the coupling of 4-methyl-5-(2-hydroxyethyl)thiazole phosphate (HET-P) and 4-amino-2-methyl-5-hydroxymethylpyrimidine diphosphate (HMP-PP) to form thiamine monophosphate (TMP, TH1), as well as the activation of thiamine to TPP (TPK). A small scheme showing the different steps in thiamine biosynthesis is given in Supplementary Fig. S2A at JXB online. Results showed that the expression of genes THI1, TH1 and TPK were upregulated in different degrees after challenged with Sclerotinia for 3 h (Fig. 2A). Correlated with the upregulation of these genes, the changes in all three forms of thiamine were also elevated (Fig. 2B), suggesting that plants increased de novo thiamine biosynthesis upon Sclerotinia infection.
Thiamine biosynthesis is correlated with the Arabidopsis response to Sclerotinia. (A, B) Changes in biosynthesis genes and contents of thiamine in Arabidopsis 3h after inoculation with Sclerotinia. (C) Lesion size on tz-1 plants inoculated with Sclerotinia for 1 d. (D) Lesion size on recovery of tz-1 plants with different concentrations of thiamine. Values represent means ±SD of three independent experiments. **P <0.01; *P <0.05 (Student’s t-test). Different letters indicate statistically significant differences (Duncan’s multiple range tests; P <0.05).
We next examined the lesion formation with the thiamine auxotrophic plant tz-1, which cannot survive on thiamine-free medium due to mutation of HET-P synthase. RP-HPLC results showed that tz-1 plants had lower levels of thiamine than Col-0 after 4 weeks grown in soil with 0.01% thiamine (Supplementary Fig. S2B). Thiamine deficiency made plants more susceptible to Sclerotinia (Fig. 2C). Unexpectedly, although exogenous application of thiamine increased the resistance of tz-1 plants, there was a relatively small reduction in lesion size compared with Col-0. This led us to doubt how far endogenous thiamine really contributed to resistance to Sclerotinia. We then tested the disease severity with different degrees of recovery of tz-1 plants that were grown in soil containing 50–300 μM thiamine after germination on agar medium weekly with 0.01% thiamine. The lesion size caused by Sclerotinia decreased with increasing of recovery of thiamine concentration in tz-1 plants (Fig. 2D and Supplementary Fig. S2C). These findings suggested that the elevated level of thiamine in Arabidopsis is correlated with enhanced resistance to Sclerotinia.
Thiamine alleviates the inhibition of Sclerotinia on host ROS burst
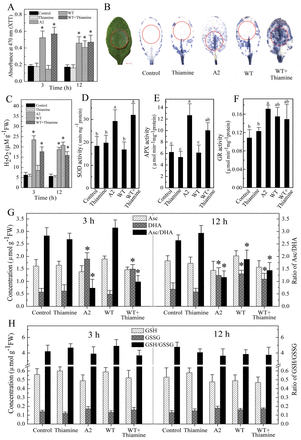
Sclerotinia-secreted oxalate can abolish host defences by suppressing ROS generation at the early stages of infection (Cessna et al., 2000). Consistent with this, wild-type Sclerotinia, but not the A2 mutant, inhibited O2– and H2O2 accumulation at 3h after inoculation. However, the wild-type Sclerotinia provoked augmented accumulation of O2– and H2O2 in leaves pre-treated with thiamine (Fig. 3A, C). Moreover, in situ staining with NBT also showed that thiamine pre-treatment increased O2– generation when challenged with wild-type Sclerotinia for 3h (Fig. 3B). Similar to the early stages, at 2 d post-inoculation with wild-type Sclerotinia, thiamine primed strong NBT staining at the leading edge of necrotic lesions (Supplementary Fig. S3A at JXB online). Additionally, wild-type Sclerotinia-induced ROS levels showed no discernible differences from samples either with or without thiamine pre-treatment at 12h (Fig. 3A, C), suggesting that thiamine-alleviated inhibition of host ROS generation occurred at the early pathogenicity stages.
Thiamine alleviates the inhibitory action of oxalate on the host’s initial cellular redox status. (A) Effect of thiamine on O2– generation in Arabidopsis upon Sclerotinia infection. (B) In situ detection of O2– formation at 3h after Sclerotinia inoculation. Circles represented the location of agar plug (diameter 0.8cm) inoculation as shown on the left-most leaf. Bar, 0.2cm. (C) Quantitative determination of H2O2 generation. (D–F) Changes in SOD (D), APX (E), and GR (F) activities after inoculation with Sclerotinia for 3h. (G, H) Changes in Asc/DHA (G) and GSH/GSSG (H) ratios in leaves treated with thiamine prior to Sclerotinia inoculation. Data are means ±SD of at least three replicates. Asterisks indicate Student’s t-test significant at P <0.05 versus the control. Different letters indicate statistically significant differences (Duncan’s multiple range tests; P <0.05). FW, fresh weight. (This figure is available in colour in JXB online.)
To investigate the effect of thiamine on the changes in cellular redox status, the activities of antioxidant enzymes (SOD, APX, and GR), and the levels of reduced (Asc, GSH) and oxidized (DHA, GSSG) ascorbate and glutathione, respectively, were measured. Both the SOD and APX activities were enhanced by thiamine in wild-type Sclerotinia-infected samples (Fig. 3D, E). However, the activities of GR did not significantly change with or without pre-treatment with thiamine (Fig. 3F). Accompany with these enzymes, thiamine pre-treatment and subsequent wild-type Sclerotinia infection triggered a reduction of the Asc/DHA ratio (Fig. 3G), reflecting an increasing oxidative status. However, the ratio of GSH/GSSG did not show significant variation under all treatments (Fig. 3H), indicating that thiamine-induced cellular redox changes in wild-type Sclerotinia-infected tissues were more specific to the Asc redox state. Additionally, at 12h post-inoculation, wild-type Sclerotinia induced a decrease in the Asc/DHA ratio, but no significant difference was observed relative to samples pre-treated with thiamine (Fig. 3G). These results suggest that pre-treatment with thiamine led to the cellular transition towards oxidative status in the infected tissues at early pathogenesis.
Activation of NOX confers thiamine-induced resistance to Sclerotinia
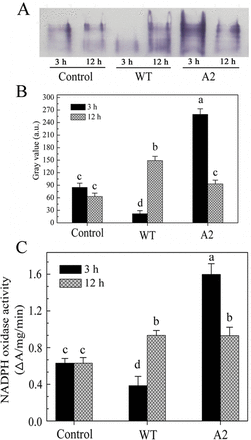
Since NOX has a dedicated function of generating ROS (Marino et al. 2012), we explored whether the activation of NOX was affected by Sclerotinia using an in situ gel NBT assay. At 3h post-inoculation, a main activity band was detected in samples infected with the A2 mutant, but only a weak band was observed with wild-type Sclerotinia (Fig. 4A). Moreover, NOX activity showed slight upregulation in wild-type Sclerotinia-infected leaves and downregulation in A2-inoculated samples after 12h of infection (Fig. 4A, B). Similar results were obtained by measuring XTT formazan to examine NOX activity (Fig. 4C). These data indicated that inhibition of NOX activity is possibly required for the oxalate-induced reducing environment in the early infection stages.
NOX is activated by the A2 mutant but inhibited by wild-type Sclerotinia at the early stages of infection. (A) In situ gel NBT assay for NOX activity. Plasma membrane vesicles were used to test the activity of NOX. (B) Grey intensity was quantified with ImageJ software. (C) The O2–-producing capabilities of NOX were measured based on XTT reduction. Data are means ±SD of three independent experiments. Differences within each treatment were evaluated by Duncan’s multiple range test (P <0.05). (This figure is available in colour in JXB online.)
To test whether NOX was involved in the signal transduction induced by thiamine, the NOX activity was analysed in leaves pre-treated with thiamine following Sclerotinia inoculation for 3h. Compared with wild-type Sclerotinia inoculation alone, thiamine pre-treatment and subsequent wild-type Sclerotinia infection increased the activity of NOX (Fig. 5A). However, this effect was abolished by the NOX inhibitor DPI (100 μM), and was concomitant with a reduced H2O2 generation (Fig. 5B). Moreover, upon wild-type Sclerotinia infection, inhibition of NOX with DPI led to a decrease in APX activity, and a reduction of the Asc/DHA ratio in leaves pre-treated with thiamine (Fig. 5C, D).
Thiamine activates NOX at the early stages of Sclerotinia infection. (A) Thiamine increased NOX activity in leaves challenged with wild-type Sclerotinia for 3h. (B) Quantitative determination of H2O2. (C) Inhibition of NOX with DPI increases the ratio of Asc/DHA. The levels of Asc and DHA were measured from wild-type Sclerotinia-infected leaves after 3h. (D) DPI weakens APX activity in samples pre-treated with thiamine. (E–G) Effect of thiamine on lesion development (E), H2O2 production (F), and APX activities (G) in NOX mutants challenged with wild-type Sclerotinia. Asterisks indicate significant differences from control or between Col-0 and the NOX mutants: **P <0.01; *P <0.05; #P <0.05 versus the indicated samples (Student’s t-test). Different letters indicate significant differences (Duncan’s multiple range test, P <0.05).
To genetically determine the role of NOX in the thiamine-induced resistance, mutants of NOX were tested for their response to Sclerotinia. Strikingly, thiamine pre-treatment could not effectively protect AtrbohD and AtrbohDF plants against Sclerotinia invasion (Fig. 5E). After 3h inoculation with Sclerotinia, thiamine-induced H2O2 generation was reduced by 41% in AtrbohD, and by 67% in the double mutant AtrbohDF (Fig. 5F). Consistently, APX activity was also significantly downregulated in both AtrbohD and AtrbohDF plants relative to Col-0 (Fig. 5G). These results indicated that thiamine could enhance NOX activity in the early pathogenesis, which might contribute to alleviate the suppression of initial host oxidative burst caused by Sclerotinia.
[Ca2+]cyt and protein kinase(s) participate in thiamine-induced activation of NOX
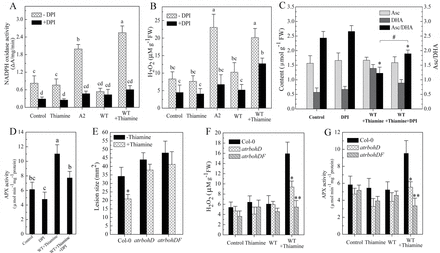
We analysed RBOHD and RBOHF gene expression to investigate whether thiamine was attributed to transcriptional regulation of NOX. Compared with the control, no major difference in expression of RBOHD or RBOHF was observed in samples pre-treated with thiamine at 3h after Sclerotinia infection (Fig. 6A). To explore the possible factors that influenced NOX activation by thiamine, we first measured the changes in [Ca2+]cyt levels with a Ca2+-sensitive fluorescent dye, Fluo-3-AM. As shown in Fig. 6B, relative to samples infected with wild-type Sclerotinia alone, the fluorescence intensity of Fluo-3 in leaves pre-treated with thiamine was significantly increased. The effect of [Ca2+]cyt on NOX activity was then investigated by Ca2+ scavengers. Thiamine-induced NOX activity was reduced by the addition of 5mM EGTA (36%, Fig. 6C), and strongly decreased in the presence of 1mM BAPTA-AM (71%, Fig. 6C). We also determined whether protein phosphorylation participated in thiamine-induced activation of NOX. Following co-treatment with 20 μM staurosporine, a broad-range protein kinase inhibitor, thiamine-induced activation of NOX was markedly inhibited (Fig. 6D). Concomitant with the decreased NOX activity, both BAPTA-AM and staurosporine weakened the initial ROS generation and the subsequent resistant effect induced by thiamine in Sclerotinia-infected leaves (Fig. 6E–G). These results suggest that Ca2+ and staurosporine-sensitive protein kinase(s) are important signal mediators required for thiamine-induced NOX activation.
[Ca2+]cyt and protein kinase(s) participate in thiamine-induced activation of NOX. (A) Changes in RBOHD and RBOHF expression in samples pre-treated with thiamine at 3h after Sclerotinia infection. (B) Thiamine-induced elevation of [Ca2+]cyt in wild-type Sclerotinia-infected leaves. (C) Removal of [Ca2+]cyt restrains thiamine-induced activation of NOX. Samples pre-treated with thiamine were sprayed with water (control), EGTA (5mM), or BAPTA-AM (1mM) prior to inoculation with wild-type Sclerotinia. (D) Staurosporine suppresses thiamine-induced activation of NOX. (E–G) Both BAPTA-AM and staurosporine abolished thiamine-induced ROS generation and resistance to Sclerotinia. Letters indicate significant differences among treatments (Duncan’s multiple range test, P <0.05).
Tissue defence responses induced by thiamine
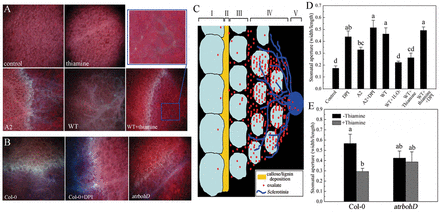
The cell wall fortification by deposition of callose/lignin is a key component of basal defence against pathogen attack (Hardham et al., 2007). We found that callose accumulation was induced by thiamine upon wild-type Sclerotinia infection. However, inhibition of NOX with DPI or mutation in AtrbohD blocked this effect (Fig. 7A, B). The tissue localization of callose is worth noting. Callose was deposited adjacent to the advancing edge of growing mycelia in A2 mutant-infected tissues. However, there was a ‘buffer zone’ between the point of thiamine-induced callose deposition and the invading fungal hyphae in leaves inoculated with wild-type Sclerotinia. Additionally, lignin was also involved in thiamine-induced cell wall reinforcement, which was further confirmed by quantifying measurement of lignin at 280nm (Supplementary Fig. S4A, Supplementary Data at JXB online). A model of cell wall fortification functions in thiamine-induced resistance to Sclerotinia is given in Fig. 7C.
Tissue defence responses induced by thiamine. (A) Thiamine-induced callose deposited around the advancing edge of necrotic areas. (B) The role of NOX in thiamine-primed callose accumulation. (C) A model of cell wall fortification functions in thiamine-induced resistance to Sclerotinia. I, healthy tissue; II, callose/lignin deposition; III, oxalate diffusion; IV, mycelia penetration; V, agar plug containing mycelia. (D, E) Thiamine-induced stomatal closure depends on NOX-derived ROS generation. Values represent means ±SD of at least 30 stomatal apertures. Differences within each treatment were evaluated by Duncan’s multiple range test (P <0.05). (This figure is available in colour in JXB online.)
Sclerotinia invasion results in foliar wilting by disturbing guard-cell functions (Guimarães and Stotz, 2004). As shown in Fig. 7C, pre-treatment with thiamine alleviated the wild-type Sclerotinia-caused increase of the stomatal aperture under darkness. Interestingly, suppression of NOX either with DPI or by mutation in AtrbohD nullified this effect (Fig. 7D, E). Conversely, exogenous application of 0.5mM H2O2 effectively promoted the stomatal closure (Fig. 7C). The results suggested that NOX-derived ROS generation is required for thiamine-induced stomatal closure in Sclerotinia-infected tissues.
Discussion
In this study, we investigated the effectiveness and mode of action of thiamine in Arabidopsis resistance against the necrotrophic pathogen Sclerotinia. To explore the mechanisms behind thiamine-induced resistance, we examined the involvement of NOX-mediated ROS signalling and the correlated cellular defence responses.
Thiamine-induced resistance against Sclerotinia is based on ROS generation
Accumulating evidence suggests that thiamine is an activator that triggers plant augmented defence responses (Ahn et al., 2005, 2007). Our data show for the first time that thiamine can effectively protect Arabidopsis against Sclerotinia (Fig. 1). Early in pathogenesis, the plants activated de novo thiamine biosynthesis in response to Sclerotinia invasion (Fig. 2A, B). Thiamine deficiency, as in the auxotroph tz-1, increased plant susceptibility to Sclerotinia, but the symptom was partially alleviated by exogenous applying thiamine (Fig. 2C). These results imply that thiamine-induced defence signalling confers plant resistance to Sclerotinia.
The role of thiamine in plant resistance and adaptation to environmental stresses is relatively complex. Although thiamine is a potent scavenger of hydroxyl radicals in vitro (Jung and Kim, 2003), its contribution to plant adaptation to abiotic stress does not directly involve chemical antioxidants (Tunc-Ozdemir et al., 2009). Moreover, Ahn et al. (2007) reported that thiamine-enhanced plant resistance is dependent on H2O2 accumulation. These seemingly incongruent characteristics are also found in riboflavin, whose induction of plant resistance requires a H2O2 burst (Azami-Sardooei et al., 2010). Upon paraquat-induced oxidative stress, supplementation with thiamine cannot fully abolish ROS accumulation (Tunc-Ozdemir et al., 2009). Interestingly, we found that thiamine partially reduced A2-induced H2O2 production, but the level was still higher than that in the control (Supplementary Fig. S5 at JXB online). It seems that thiamine more likely functions as a regulator to maintain ROS generation, and keeps it at a relatively reasonable level to induce plant adaptation. Further investigations are needed to address this possibility.
The initial oxidative status is crucial for disturbing Sclerotinia infection
The cellular redox adjustments are central for defence responses during the early stages of Sclerotinia infection (Hegedus and Rimmer, 2005; Bolton et al., 2006). The early pathogenicity stage involves production of oxalate to generate a reducing environment that suppresses the host’s oxidative burst (Cessna et al., 2000). Reduction of oxalate with the A2 mutant induces high levels of H2O2 in infected tissues, whereas induction of an artificial reducing environment nullifies this effect (Williams et al., 2011). Here, we observed that thiamine pre-treatment and subsequent Sclerotinia infection rapidly induced ROS production (Fig. 3A–C). Interestingly, at the later stages of wild-type Sclerotinia infection, no significant difference in ROS generation was observed in samples either with or without thiamine pre-treatment (Fig. 3A, C). Thus, reversion of the initial cellular reducing status may be an effective mechanism for thiamine to disturb the establishment of infection of this fungus.
The interaction between ROS and antioxidants is critical for maintaining cellular redox homeostasis (Foyer and Noctor, 2005, 2011). Wild-type Sclerotinia challenged on plants pre-treated with thiamine resulted in reduced Asc redox state, but no significant change in the GSH redox state (Fig. 3G, H). In particular, the alteration of Asc redox state was correlated with the changes in H2O2 content and APX activity (Fig. 3C, E). Nevertheless, alone among the expression of antioxidative enzymes, APX1 transcript levels are not elevated in unstressed tz-1 plant (Tunc-Ozdemir et al., 2009). Thiamine may therefore alter the Asc redox state by upregulation of APX activity (Fig. 3E), which may partly account for the susceptibility of tz-1 plant to Sclerotinia (Fig. 2C). Together, these data illustrate that the reduced Asc redox is a key determinant of thiamine-reversed initial reducing status.
Activation of NOX is involved in thiamine-induced initial oxidative status
NOX has been reported in plant resistances to Sclerotinia (Guo and Stotz, 2010; Perchepied et al., 2010). Inhibition of NOX with DPI interferes with oxalate-induced PCD in plants (Kim et al. 2008). Here, we found that NOX activity was inhibited by wild-type Sclerotinia but not the A2 mutant at the initial stages of infection (Fig. 4). It is possible that Sclerotinia-secreted oxalate inhibits the activity of NOX, thus contributing to the reducing environment establishment in early pathogenesis. Thiamine could effectively alleviate the inhibition of NOX, leading to H2O2 generation and oxidative status transition in infected tissues. However, these effects were nullified by suppressing NOX activation with pharmacological and genetic approaches (Fig. 5). These results prove that NOX participates in thiamine-induced initial oxidative status upon Sclerotinia infection.
Although we observed that thiamine could enhance NOX activity, the detailed mechanisms downstream of thiamine remains incomplete. Thiamine pre-treatment did not significantly induce change of RBOHD or RBOHF expression at 3h after Sclerotinia infection (Fig. 6A), suggesting that thiamine perhaps is not attributed to transcriptional regulation of NOX at the early stages of infection. Ahn et al. (2005) reported that thiamine could promote fluxion of Ca2+ and upregulation of protein kinase C-like activity. Interestingly, these two factors are critical signals upstream of activating NOX activity (Quagliaro et al., 2003). Consistently, both BAPTA-AM and staurosporine inhibited NOX activity and the subsequent ROS generation in samples pre-treated with thiamine upon Sclerotinia infection (Fig. 6C–F). These results indirectly demonstrated that Ca2+ and staurosporine-sensitive protein kinase(s) participated as important signal mediators in thiamine-induced activation of NOX.
In addition, whether thiamine can interact with multiple phytohormones (e.g. ABA) during the process of NOX activation is unknown. ABA-regulated NOX has been reported in plant resistance to external stresses (Jiang and Zhang, 2002). Recently, we found that Sclerotinia could reduce ABA levels by causing a dysfunctional xanthophyll cycle at the initial stages of infection (unpublished data). Interestingly, exogenous application of ABA can increase the transcripts and contents of thiamine (Rapala-Kozik et al., 2012). It will be worth studying thiamine–ABA interactions during the process of NOX activation in future research.
Tissue defence response induced by thiamine requires activation of NOX
Upon pathogen attack, infected cells respond by local reinforcement of the cell wall by rapidly forming callose and lignin (Ton and Mauch-Mani, 2004; Hardham et al., 2007). Tu (1985) reported that the rate of oxalate diffusion in leaf tissue is paralleled by the plant’s susceptibility. However, an oxalate-deficient mutant was non-pathogenic to plants (Godoy et al., 1990). Thus, cell wall fortifications with callose/lignin might be an effective barrier to prevent oxalate diffusion. Interestingly, the activation of NOX was necessary for thiamine-primed callose/lignin (Fig. 7A, B, and Supplementary Fig. S4), which were deposited at similar sites to O2– accumulation in the early infected tissues (Supplementary Fig. S3). Thiamine might induce callose/lignin deposition to prevent oxalate diffusion and subsequent mycelium penetration via NOX-mediated ROS signalling. Additionally, thiamine-induced stomatal closure was also closely correlated with NOX-derived ROS generation, which might help Sclerotinia-infecteded plants protect against foliar wilting (Fig. 7D, E).
Besides the Arabidopsis model, analysis of the effect of thiamine in the context of the crop–Sclerotinia interaction would give some interesting clues about whether the regulation is conserved. Excitingly, thiamine pre-treatment enhanced the resistance to Sclerotinia in both cultivars of susceptible B. napus ‘Yinong 34’ and the partially resistant ‘Zhongshuang 9’ (Supplementary Fig. S6 at JXB online). Moreover, thiamine-induced resistant effects including increased NOX activity, ROS generation, and callose accumulation were also found in B. napus upon Sclerotinia inoculation. Interestingly, the cultivar ‘Zhongshuang 9’ showed higher production of H2O2 compared with the susceptible cultivar ‘Yinong 34’ upon Sclerotinia infection (Supplementary Fig. S6D). Consistently, similar results were also found in the resistant Arabidopsis ecotypes Col-0 and Rbz-1, which generated higher levels of H2O2 than the susceptible ecotype Sha in response to Sclerotinia (Perchepied et al., 2010). These data prove that the initiation of an oxidative burst is required for enhancing plant resistance to Sclerotinia.
In summary, our results contribute to elucidation of the defence mechanisms induced by thiamine in plant resistance to Sclerotinia. We have provided evidence that interfering with the establishment of an initial reducing status is crucial in impeding the pathogenesis of Sclerotinia. Early in pathogenesis, thiamine could effectively alleviate the inhibitory action of Sclerotinia on NOX activity, which promotes ROS generation and follows a decreased Asc/DHA ratio, reflecting the cellular transition towards oxidative status in infected tissues. Of particular interest is the localization of callose/lignin in infected tissue. Since oxalate diffusion in leaf tissue is crucial for the pathogenesis of Sclerotinia (Tu, 1985), we presume that thiamine might induce cell wall fortifications with callose/lignin to prevent oxalate diffusion and disturb pathogen compatibility by activating NOX-mediated ROS signalling.
Acknowledgements
We thank Professor Martin Dickman (Texas A&M University, USA) for providing the strains of Sclerotinia, and Professor Hanzhong Wang (Oil Crops Research Institute, China) for providing B. napus ‘Zhongshuang 9’. This research is supported by the Program for Changjiang Scholars and Innovative Research Team in University (IRT0829), the Key Program of NSFC-Guangdong Joint Funds of China (U0931005), and the National High Technology Research and Development Program of China (863 Program) (2007AA10Z204).
Abbreviations:
- ABA
abscisic acid
- APX
ascorbate peroxidase
- DPI
diphenyleneiodonium
- GR
glutathione reductase
- NBT
nitroblue tetrazolium
- NOX
NADPH oxidase
- O2–
superoxide anion
- PCD
programmed cell death
- qRT-PCR
quantitative real-time PCR
- ROS
reactive oxygen species
- RP-HPLC
reverse-phase high-pressure liquid chromatography
- SD
standard deviation
- SOD
superoxide dismutase
- TPP
thiamine pyrophosphate
- XTT
3ʹ-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]-bis(4-methoxy-6-nitro) benzene sulfonic acid hydrate.
References



![[Ca2+]cyt and protein kinase(s) participate in thiamine-induced activation of NOX. (A) Changes in RBOHD and RBOHF expression in samples pre-treated with thiamine at 3h after Sclerotinia infection. (B) Thiamine-induced elevation of [Ca2+]cyt in wild-type Sclerotinia-infected leaves. (C) Removal of [Ca2+]cyt restrains thiamine-induced activation of NOX. Samples pre-treated with thiamine were sprayed with water (control), EGTA (5mM), or BAPTA-AM (1mM) prior to inoculation with wild-type Sclerotinia. (D) Staurosporine suppresses thiamine-induced activation of NOX. (E–G) Both BAPTA-AM and staurosporine abolished thiamine-induced ROS generation and resistance to Sclerotinia. Letters indicate significant differences among treatments (Duncan’s multiple range test, P <0.05).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/64/11/10.1093_jxb_ert166/2/m_exbotj_ert166_f0006.gif?Expires=1716385863&Signature=Ew7Nb~wvTZy6-DR6QIRK7WOUMNBYlvrZG6YCix75nNb6FlYsKXy14vEGHPWFBc37iwYEp42ZOfIOP0jcrp1~cAKgeinE7iyUzuiLuQOvjR2EsUOkECyoq8MdRlhnAEFNc~TWT~D73oBl-RU6-RT0UnYF16dDXBrh-AXFOh2eAdY2SWNFpgjx1giCROvyiA-GZFseLZekZ2k-Ms~yu0gV9zRgCk-NW51ccIkMfzoOOTlpso~lsw8meD-MrbJouT2636R8pRysk7whf8W6eFiuc0RTKlm6MEpWLwzd2RXtVEX-cNjsSSzj4eppqNA92BPHuLO1I06gmT74XhV4byOH9A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

Comments