-
PDF
- Split View
-
Views
-
Cite
Cite
Aart J. E. van Bel, Alexandra C. U. Furch, Torsten Will, Stefanie V. Buxa, Rita Musetti, Jens B. Hafke, Spread the news: systemic dissemination and local impact of Ca2+ signals along the phloem pathway, Journal of Experimental Botany, Volume 65, Issue 7, April 2014, Pages 1761–1787, https://doi.org/10.1093/jxb/ert425
Close - Share Icon Share
ABSTRACT
We explored the idea of whether electropotential waves (EPWs) primarily act as vehicles for systemic spread of Ca2+ signals. EPW-associated Ca2+ influx may trigger generation and amplification of countless long-distance signals along the phloem pathway given the fact that gating of Ca2+-permeable channels is a universal response to biotic and abiotic challenges. Despite fundamental differences, both action and variation potentials are associated with a sudden Ca2+ influx. Both EPWs probably disperse in the lateral direction, which could be of essential functional significance. A vast set of Ca2+-permeable channels, some of which have been localized, is required for Ca2+-modulated events in sieve elements. There, Ca2+-permeable channels are clustered and create so-called Ca2+ hotspots, which play a pivotal role in sieve element occlusion. Occlusion mechanisms play a central part in the interaction between plants and phytopathogens (e.g. aphids or phytoplasmas) and in transient re-organization of the vascular symplasm. It is argued that Ca2+-triggered systemic signalling occurs in partly overlapping waves. The forefront of EPWs may be accompanied by a burst of free Ca2+ ions and Ca2+-binding proteins in the sieve tube sap, with a far-reaching impact on target cells. Lateral dispersion of EPWs may induce diverse Ca2+ influx and handling patterns (Ca2+ signatures) in various cell types lining the sieve tubes. As a result, a variety of cascades may trigger the fabrication of signals such as phytohormones, proteins, or RNA species released into the sap stream after product-related lag times. Moreover, transient reorganization of the vascular symplasm could modify cascades in disjunct vascular cells.
Introduction
In their natural habitat, plants are permanently exposed to countless abiotic and biotic changes imposing a permanent stress. The majority of environmental challenges are communicated via the extracellular microenvironment (i.e. the apoplasmic space) and, from there, via the plasma membrane to the intracellular space. The external stimuli are monitored by a vast battery of sensors which transform the external information into signals triggering adequate cell reactions. One of the initial events in sensing is a Ca2+ influx modulated by Ca2+-permeable channels at the plasma membrane. Since no Ca2+-selective channels have been identified with certainty in the plasma membrane of plant cells thus far (Kudla et al., 2010), we will refer to these channels as ‘Ca2+-permeable’ (e.g. Sanders et al., 2002). Ca2+ influx elevates the cytosolic Ca2+ level according to stimulus-specific, spatio-temporal, and potentially cell-specific patterns designated Ca2+ signatures (Webb et al., 1996; Ng and McAinsh, 2003; McAinsh and Pitman, 2009; Kudla et al., 2010). These signatures are decoded and relayed by signalling molecules into a multitude of downstream events.
Ca2+ influx is associated with a depolarization that can propagate electrotonically via plasmodesmata towards other cells (e.g. Brinckmann and Lüttge, 1974; Overall and Gunning 1982; van Bel and van Rijen, 1994; Holdaway-Clarke et al., 1996). The depolarization range is mostly limited to a few cells due to the electrical resistance of plasma membranes and plasmodesmata (Overal and Gunning, 1982). However, influx of Ca2+ can initiate so-called receptor potentials which trigger action potentials (e.g. Benolken and Jacobson, 1970; Williams and Pickard, 1972b), provided that adjacent cells have become specialized in creating self-amplifying potential waves that can cover appreciable distances. Examples of non-vascular propagation of electropotential waves (EPWs) are the pollen-triggered (Sinyukin and Britikov, 1967) or temperature-triggered effects (Fromm et al., 1995) on ovary respiration. During evolution, conduction of EPWs has been perfected in sieve tubes owing to a reduced resistance of the symplasmic connections in the transverse walls to facilitate mass flow through sieve pores (Fromm and Lautner, 2012; Hafke and van Bel, 2013). The increased pore diameters, the high saline content, and the ability to propagate self-amplifying potential waves made sieve tubes ideal pathways for electrical long-distance signalling (Samejima and Sibaoka, 1983; Fromm, 1991; Wildon et al., 1992; Fromm and Spanswick, 1993; Rhodes et al., 1996; Fromm and Lautner, 2006; Furch et al., 2007).
As mentioned above, Ca2+ influx triggers a multidimensional web of intracellular cascades leading to changes in metabolism and/or gene expression, thereby conferring adaptive reactions (Sanders et al., 2002; White and Broadley, 2003; McAinsh and Pittman, 2009; Kudla et al., 2010). Because visibly non-excitable plants also dispose of the capacity for electrical systemic signalling (Fromm and Lautner, 2012; Hafke and van Bel, 2013; Zimmermann and Mithöfer, 2013), the question arises of whether EPWs have universal functions—associated with Ca2+ influx—in inter-organ communication. Perhaps electrical long-distance signals in plants primarily serve to distribute Ca2+ signals rapidly (e.g. Pyatygin et al., 2008; van Bel et al., 2011a), providing a common platform for several chemical forms of long-distance signalling. In this manner, EPWs may act as vehicles for distribution of Ca2+ as the ‘lead currency’ in plant signalling (Kudla et al., 2010). Therefore, we will explore the impact of EPWs on the systemic distribution of Ca2+ influx and the inherent responses of diverse cell types along and at the termini of the phloem pathway.
Electropotential waves
Short descriptions and definitions
Diverse types of EPWs propagate along the vascular pathways (Pickard, 1973; Rhodes et al., 1996; Stahlberg and Cosgrove, 1997; Davies, 2006; Davies and Stankovic, 2006; Stahlberg et al., 2006; Furch et al., 2007; Hafke et al., 2009; Zimmermann et al., 2009; Zimmermann and Mithöfer, 2013). Action potentials (APs) are transmitted more rapidly (0.5–10cm s–1) than variation potentials (0.1–1.0cm s–1) (VPs) (Stahlberg and Cosgrove, 1997; Fromm and Lautner, 2007), while the propagation velocity of the recently proposed system potentials (SPs; Zimmermann et al., 2009) is 5–6cm min–1.
Strong injuries such as burning trigger both APs and VPs, resulting in composite signals (Houwink, 1935; van Sambeek and Pickard, 1976; Roblin, 1985; Roblin and Bonnemain, 1985; Stankovic et al., 1997, 1998; Hlavackova et al., 2006), when recorded in the close vicinity of the burning site (Davies and Stankovic, 2006; Furch et al., 2007, 2009; Hafke et al., 2009). With increasing distance, the signals drift apart due to the different propagation velocities (Davies et al., 1991; Stankovic et al., 1998; Davies and Stankovic, 2006; Furch et al., 2010), which implies that EPW profiles can differ between various locations along the propagation pathway.
A proper assessment of EPW profiles requires some further awareness of the caveats in recording. Overlapping APs and VPs giving rise to ‘mixed’ EPWs often impede proper signal analysis of the AP and VP component. Furthermore, EPW types are distinguished on the basis of kinetic parameters such as amplitude, duration, and profile (e.g. Stahlberg et al., 2006). However, kinetic features alone (Stankovic et al., 1997; Dziubinska et al., 2001) might be a doubtful criterion for a distinction between APs and VPs, because VPs often mimick AP kinetics (Stahlberg et al., 2006; Furch et al., 2008; Fromm et al., 2013). For instance, cutting often causes VPs that have a close similarity to APs, while crushing results in long-lasting VPs (van Sambeek and Pickard, 1976).
Action potentials
APs are mostly associated with non-invasive stimuli (Stahlberg et al., 2006; Trebacz et al., 2006). Examples of AP-induced events propagating along sieve tubes are the fast thigmonastic movements in touch-sensitive plants (Sibaoka, 1969, 1991), the touch-induced closure of the Venus flytrap (Sibaoka, 1966; Hodick and Sievers, 1989), the movements of the waterwheel plant (Iijima and Sibaoka, 1981), and the complex bending of sundew leaf tentacles (Williams and Pickard, 1972a, b, 1974; Williams and Spanswick, 1976). APs are also induced by dipping leaves in ice-cold water (Fromm, 1991; Fromm and Bauer, 1994) as well as by drought-mimicking treatments of roots (Fromm and Fei, 1998).
APs are characterized by spike-like changes of the membrane potential (Stahlberg and Cosgrove, 1997). A period of >1min between the onset of the depolarization and completion of the repolarization usually identifies an EPW as an AP (Pickard, 1973; Fromm, 1991). As in neurons, APs seem to be all-or-nothing events (Fromm and Spanswick, 1993; Pyatygin et al., 2008). Below a critical membrane potential threshold, APs or self-amplifying depolarizations start their propagation with defined amplitudes and velocities independent of the stimulus strength (Fromm, 1991; Zawadzki et al., 1991; Wildon et al., 1992; Fromm and Spanswick, 1993; Davies and Stankovic, 2006; Fromm and Lautner, 2007).
AP kinetics depend on the integrated activities of voltage-dependent ion channels in the plasma membrane (Lunevsky et al., 1983; Okihara et al., 1991; Zawadzki et al., 1991; Fromm and Bauer, 1994; Wayne, 1994; Davies, 2004). The ionic exchanges during APs (Trebacz et al., 2006) seem to bear a strong resemblance throughout the entire plant kingdom (Iijima and Sibaoka, 1981, 1982, 1985; Hodick and Sievers, 1988; Fromm and Spanswick, 1993; Fromm and Bauer, 1994; Trebacz et al., 1994; Opritov et al., 2002; Krol et al., 2003, 2004; Fisahn et al., 2004; Felle and Zimmermann, 2007) and in some algal taxa (Lunevsky et al., 1983; Okihara, 1991; Homann and Thiel, 1994; Thiel et al., 1997).
The ion channels involved differ from those engaged in stimulus conduction along nerve cells. An initial depolarization is associated with a transient increase in Ca2+ concentration mediated by stimulus-triggered gating of Ca2+-permeable channels in the plasma membrane (Fig. 1). The elevated Ca2+ level brings about Cl– efflux through Ca2+-dependent anion channels also located in the plasma membrane (Lunevsky et al., 1983; Tsutsui et al., 1986; Okihara et al., 1991; Homann and Thiel, 1994). Voltage-dependent K+ channels are gated just before the steady-state potential for Cl– ions is reached, which causes K+ efflux to repolarize the membrane potential (Homann and Thiel, 1994; Thiel et al., 1997). Since full repolarization is limited by the Nernst potential of K+, re-establishment of the resting potential is achieved by auxiliary input of proton pumps (Kishimoto et al., 1985).
Diagram of the Ca2+ influx events associated with action potentials (APs) and variation potentials (VPs). APs, which are rolling as longitudinal waves along the sieve tubes, are triggered by various non-invasive stimuli leading to a domino-like Ca2+ influx (upper row of blue arrows). VPs are triggered by relaxation of the negative hydrostatic pressure in xylem vessels, giving rise to water uptake (green arrows) in the adjacent vascular parenchyma cells. Turgor-induced tensile forces stimulate Ca2+ influx and an inherent receptor potential that relays information to the sieve element.
Variation potentials
VPs are mostly triggered by damaging treatments such as burning (Houwink, 1935; van Sambeek and Pickard, 1976; Roblin, 1985; Wildon et al., 1992; Stankovic et al., 1998; Mancuso, 1999; Furch et al., 2007, 2010; Hafke et al., 2009), hot water (van Sambeek and Pickard, 1976), vigorous cutting (Mancuso, 1999), strong crushing (van Sambeek and Pickard, 1976), and injury by chewing insects (Alarcon and Malone, 1994).
VPs do not obey the all-or-nothing law: the signals are positively correlated with the stimulus strength and last for from 10 s up to 30min (Stahlberg and Cosgrove, 1997; Stahlberg et al., 2006). The propagation velocities are 5–10 times slower than those of APs (Stahlberg and Cosgrove, 1997) and the amplitude drops along the transmission path (Davies, 2004; Stahlberg et al., 2005, 2006). As a result, VP amplitudes decrease with increasing distance from the stimulus site and finally extinguish (van Sambeek and Pickard, 1976).
The slow repolarization of VPs might result from the shutdown of proton pumps as indicated by pH-dependent fluorochromes and the ineffectiveness of ion channel blockers (Stahlberg and Cosgrove, 1992, 1996). The inhibition of proton pump activity is not fully understood, but may be due to elevated Ca2+ levels (Kinoshita et al., 1995; Hafke et al., 2013). Proton pump activity may be equally suppressed by cytosolic Ca2+ in APs, but the reduced pump activity may be hardly detectable due to the lower Ca2+ influx during APs (see ‘Creation of Ca2+ hotspots in sieve elements’).
Generation and propagation of VPs have only been observed in intact plants, whilst APs can propagate in isolated organs (Stahlberg et al., 2006). Therefore, relaxation of the negative hydrostatic pressure in the xylem vessels is the likely source of VP generation (Stahlberg and Cosgrove, 1997). That VPs originate from events in xylem vessels (Fig. 1) was demonstrated by the fact that VPs, in contrast to APs, are able to traverse dead or poisoned areas (Stahlberg et al., 2006).
Essential differences and functional similarities between APs and VPs
The preceding sections disclose a few essential differences between the APs and VPs (Fig. 1). (i) APs and VPs are of a dissimilar nature (i.e. they are of an electrical or mechanistic origin) and, by implication, the Ca2+-permeable channels responsible for the initial depolarization are either voltage-dependent or mechano-sensitive channels, respectively (Fig. 1). (ii) APs are generated in non-vascular or vascular cells, move longitudinally along the sieve tubes, and may disperse in the lateral direction to surrounding vascular cells (Fig. 1). In contrast, VPs are generated by vascular (xylem parenchyma) cells and move laterally across several cell layers to the sieve tubes, so that VPs reflect arrival of successive single depolarizations at the sieve element plasma membrane mimicking electrical propagation along the sieve tubes (Fig. 1; Malone, 1996; Pyatygin et al., 2008; van Bel et al., 2011a).
These conclusions call for further exploration of the following questions: (i) which Ca2+-permeable channels reside in the vascular cells and where are they located (Fig. 2); and (ii) how does the symplasmic organization of the phloem strands enable combined longitudinal propagation and lateral dispersion of electrical information (Fig. 3)?
Ca2+-permeable channels localized to the plasma membrane in plants. According to McAinsh and Pittman (2009), the respective groups are: MSCs (mechano-sensitive channels), DACCs (depolarization-activated channels), HACCs (hyperpolarization-activated channels), GLRs (glutamate receptor-like channels) and CNGCs (cyclic nucleotide-gated channels). (B–E) Confocal images of the distribution of Ca2+-permeable channels in intact sieve elements (from Furch ACU, van Bel AJE, Fricker MD, Felle HH, Fuchs M, Hafke JB. 2009. Sieve element Ca2+ channels as relay stations between remote stimulus and sieve tube occlusion. The Plant Cell 21, 2118–2132. www.plantcell.org. Copyright American Society of Plant Biology). (B) Localization of Ca2+-permeable channels by BODIPY-DHP (red). Ca2+ channels are mostly aggregated (white arrowheads) against the sieve element wall facing the companion cell (CC) and in the vicinity of the sieve plates (see insets). (C) False-colour image of Ca2+-permeable channels localized to the plasma membrane of sieve elements in situ obtained by the ratio of the measured fluorescence of BODIPY-DHP and RH-414. Agglomerates of Ca2+ channels occur near the sieve plates (SPs) and the plasmodesmal contacts with CCs (white arrowheads; PPC, phloem parenchyma cell). (D) Localization of Ca2+-permeable channel distribution (stained by BODIPY-DHP, green) along the sieve element plasma membrane (stained by RH-414, red). Green dots indicate an uneven distribution of Ca2+-permeable channels (Furch et al., 2009) similar to the distribution of ER stacks (Ehlers et al., 2000). (E) Co-localization (yellowish) of Ca2+-permeable channels (stained by BODIPY-DHP) and ER membranes (stained by ER-tracker Red) along the sieve element (SE) wall facing the CCs (small arrowheads) and close (large arrow heads) to the sieve plate (SP, arrow).
Potential lateral modes of EPW dispersion. The upper row of symbols represents an action potential propagation by successive gating of Ca2+-permeable, Cl– and K+ channels. (A) Lateral electrotonic displacement of an action potential via activation of voltage-dependent (violet circles). (B) Lateral electrotonic displacement of a variation potential in a direction opposite to that in A via activation of voltage-dependent (violet circles). (C) Lateral displacement of a variation potential via activation of MSCs (lilac circles). (D) Lateral displacement of a variation potential via a combined action of MSCs (lilac circles) triggering ligand-producing cascades (stippled line) and ligand-activated Ca2+-permeable channels (dark blue circles).
Despite their profound differences, EPWs have one essential, functional feature in common. They are all associated with an initial elevation of cytosolic Ca2+ (Trebacz et al., 2006; Davies and Stankovic, 2006; Demidchik and Maathuis, 2007; McAinsh and Pittman, 2009), regardless of the involvement of voltage-dependent, mechano-sensitive, or ligand-activated Ca2+-permeable channels. Hence, the elevation of Ca2+ levels in sieve elements, the involvement of the sieve element cytoskeleton in Ca2+ influx mechanisms, and the impact of Ca2+ influx on sieve element biology are major issues in this frame (Figs. 4–6).
Use of forisomes as indicators of Ca2+ concentration thresholds and the formation of Ca2+ hotspots in legume sieve tubes. (A) Microelectrode-recorded passage of an EPW triggered by remote burning (from Furch ACU, Hafke JB, Schulz A, van Bel AJE. 2007. Ca2+-mediated remote control of reversible sieve tube occlusion in Vicia faba. Journal of Experimental Botany 58, 2827–2838 with permission of the Society for Experimental Biology) in a sieve element. The arrows (B–E) indicate the time points at which confocal laser-scanning images of forisomes (asterisk) in the sieve element (SE) were taken. Condensed forisomes are visible (B and E), but dispersed forisomes are not (C and D) due to changed optical properties. The whitish spots are autofluorescent chloroplasts. Companion cells (CCs) and sieve plates are marked with arrowheads. (F–I) The effect of Ca2+ channel inhibitors on forisome dispersion (Furch et al., 2009) in response to remote burning (see B, C as controls). (F and G) Absence of forisome dispersion (asterisk) in the presence of La3+. Black arrowsheads point to electrode tips. (H and I) Absence of forisome dispersion (asterisk) in the presence of nifedipine. (J) The creation of Ca2+ hotspots. Hypothetical deployment of Ca2+-permeable channels, the ‘EPW strength’, and the influx of Ca2+ ions (strongly modified after Hafke JB, Furch ACU, Fricker MD, van Bel AJE. 2009. Forisome dispersion in Vicia faba is triggered by Ca2+ hotspots created by concerted action of diverse Ca2+ channels in sieve elements. Plant Signaling and Behavior 4, 968–972). This model assumes that a graded stimulus–response correlation depends on the increasing engagement of diverse Ca2+-permeable channels with increasing stimulus strength. The latter results in cumulative Ca2+ release into the unstirred mictoplasm. The impact of the stimulus increasing from a to d is depicted by profile symbols representing the contribution of APs and VPs to the EPW and the putative Ca2+ influx. The grey tones demonstrate Ca2+ concentrations effected by various stimuli in the vicinity of ER–plasma membrane conglomerates which are connected by nano-anchors (see Fig. 5F). (a) Small AP-like depolarizations induce minute release of Ca2+ ([Ca2+]mict stippled arrow) via voltage-dependent Ca2+ channels (green symbols) into the mictoplasm. These may affect forisome attachment given the slight forisome movements, but are insufficient to bring about conformational changes of the forisome. (b) Larger AP- and VP-associated depolarizations enhance the micoplasmic Ca2+ level [Ca2+]mict to such an extent that forisomes move and/or swell. The voltage drops along the sieve element plasma membrane may be propagated via the minute protein anchors to the ER, where voltage-dependent Ca2+ channels are activated. The latter channels (green symbols) in the ER are needed to sustain the rise in Ca2+. Alternatively, or additionally, Ca2+-dependent Ca2+ channels may be activated to initiate Ca2+ release (CICR channels, yellow symbols) from the ER as reported for storage vacuoles (Bewell et al., 1999). This implies that Ca2+ ions are not released from ER cisternae without a preceding plasma membrane trigger. (c) Since cutting close to the site of observation causes a massive loss of turgor, a variation potential most probably overlaid with an AP transient induces Ca2+ release to an extent that causes full forisome dispersion. (d) A steep AP followed by a massive VP induces not only forisome dispersion, but also callose deposition. It is unclear if the VP is initiated by suppression of the plasma membrane proton pump activity leading to activation of voltage-gated channels or by direct influx via MSCs (red symbols) supplemented by Ca2+-activated Ca2+-permeable channels (yellow symbols). To elevate Ca2+ concentrations ([Ca2+]mict, thick arrow) further to the level required for callose synthesis, the Ca2+ signal may be amplified by involvement of signal molecules such as IP3. High-affinity IP3-binding sites on the ER suggest the presence of IP3-gated Ca2+ release channels (blue symbols). An increase in cellular IP3 levels has been reported in response to osmotic shocks (Munnik and Vermeer, 2010) and burning stimuli (Davies, 2004).
Involvement of actin in Ca2+-permeable channel gating. (A) Inhibitory effect of La3+ on cold shock-induced depolarization of sieve elements. (B) Inhibitory effect of latrunculin (LatA) on cold shock-induced depolarizations of sieve elements. (C) Immunolocalization (black dots) of actin located at the sieve element plasma membrane. (D) Immunolocalization (black dots) of actin localized to a forisome. (E) Confocal laser scanning image of a sieve element actin meshwork stained by microinjected fluorescent phalloidin limited by two sieve plates (sp) due to sieve plate occlusion in response to microelectrode impalement (Knoblauch and van Bel, 1998). The penetration site of the electrode (arrow) and the dispersed forisome (asterisk) are stained non-specifically. (A–E from Hafke JB, Ehlers K, Foller J, Holl S-R, Becker S, van Bel AJE. 2013. Involvement of the sieve element cytoskeleton in electrical responses to cold shocks. Plant Physiology 162, 707–714. www.plantphysiol.org. Copyright American Society of Plant Physiologists) (F) Putative interconnection between Ca2+-permeable channels and actin filaments. Three types of Ca2+-permeable channels (voltage-dependent, violet; ligand-activated, light blue; mechano-sensitive, red) are involved in Ca2+ influx into sieve elements. Ca2+-permeable channels are proposed to be incorporated into large protein complexes (transducons, dark blue; Trewavas and Malho, 1997) that are linked to the actin meshwork via NETWORKED proteins (Deeks et al., 2012). ER stacks are tethered to the plasma membrane and to each other by protein clamps (Ehlers et al., 2000). Forisome tips may also be attached to the plasma membrane by anchors of unknown composition.
Sieve element occlusion mechanisms. (A–C) Time course of callose deposition (reddish white fluorescence obtained with aniline blue) in sieve elements after remote burning. After a rapid build-up, callose is degraded more slowly at rates dependent on the stimulus strength and plant species (from Furch ACU, Hafke JB, Schulz A, van Bel AJE. 2007. Ca2+-mediated remote control of reversible sieve tube occlusion in Vicia faba. Journal of Experimental Botany 58, 2827–2838, with permission of the Society of Experimental Botany). The intense lenticular staining marks callose deposits near the sieve plate; the lateral dots mark callose deposition around the pore–plasmodesma units (PPUs). (D) Dispersion and recondensation time windows of forisome behaviour (red rectangles) superimposed on the time course of callose synthesis in legume sieve elements. Callose deposition on the PPUs (SE, right; SE, left) disappears more quickly than that on the sieve plate (SP). (E) Resting structure of legume sieve elements (SEs) with Ca2+-permeable channels (red circles) near PPUs and sieve plates, a condensed forisome, and a low degree of callose deposition (blue areas). After an increase in the Ca2+ concentration in sieve elements (e.g. after activation of the Ca2+-permeable channels by an EPW), the Ca2+ level (graded red tone) reaches a threshold (II) for forisome dispersion or even the threshold (I) for callose synthesis.
Phloem-associated Ca2+-permeable channels and other channels involved in EPW propagation
Types of Ca2+-permeable channels localized to the plasma membrane in plants
In animals, highly selective Ca2+ channels are responsible for Ca2+ fluxes at the plasma membrane (Tsien et al., 1987; Tsien and Tsien, 1990; McAinsh and Pittman, 2009), whereas non-selective cation channels (NSCCs) or Ca2+-permeable channels seem to enable Ca2+ fluxes in plants to give rise to stimulus-specific Ca2+ signatures (Demidchik and Maathuis, 2007; McAinsh and Pittman, 2009). Ca2+ influx across the plasma membrane can be mediated by the following types of non-specific cation channels (Fig. 2A; Demidchik and Maathuis, 2007; McAinsh and Pittman, 2009): (i) HACCs: hyperpolarization-activated Ca2+-permeable channels, which are gated by an increase in membrane voltage, reactive oxygen species (ROS), and changes in the cytoplasmic Ca2+ level; (ii) DACCs: depolarization-activated Ca2+-permeable channels activated by a decrease in membrane voltage; (iii) MSCs: mechano-sensitive channels the gating of which is modulated by tensile forces exerted on membranes; (iv) CNCGs: cyclic nucleotide-gated channels activated by binding of cyclic nucleotides (e.g. cAMP, cGMP); and (v) GLRs: glutamate receptor-like channels activated by binding of amino acids.
Regarding HACCs (in Arabidopsis root cells), the resting value of the membrane potential is more positive than their activation voltage, which, however, can shift to more positive membrane potentials brought about by increased Ca2+ levels (Demidchik et al., 2002; Demidchik and Maathuis, 2007; Miedema et al., 2008).
It is likely that DACCs are engaged in cold-induced Ca2+ influx (White, 2009). A member of the DACCs, named the maxi cation channel, was postulated to be responsible for the creation of complex temperature-dependent Ca2+ signatures (White and Ridout, 1999; White, 2004, 2009).
Apart from their gating response to changing tensile forces (Demidchik and Maathuis, 2007), MSCs may act as primary temperature sensors (Minorsky and Spanswick, 1989; Monroy and Dhindsa, 1995; Plieth et al., 1999), as demonstrated by the gradually increasing activity of MSCs at temperatures dropping below 20 °C (Ding and Pickard, 1993).
Putative Ca2+-permeable channels lining the sieve elements
Among all possible Ca2+-permeable channels, MSCs have been identified with certainty in the sieve element plasma membrane thus far. Forisome reactions in intact sieve elements (Knoblauch et al., 2001) and in sieve element protoplasts (Hafke et al., 2007) evidenced Ca2+ influx in response to vigorous turgor changes. MSCs may also be crucial players in the activation of HACCs that may catalyse a long-lasting Ca2+ influx into sieve elements during the prolonged EPW phase after remote burning (Furch et al., 2009). Recently, GLRs have been discovered in the phloem (Vincill et al., 2013), but their cellular location is uncertain.
Only circumstantial evidence has been obtained for other Ca2+-permeable channels in sieve tubes. Cold-shock induced Ca2+ influx into sieve elements (Thorpe et al., 2010; Hafke et al., 2013) could have been mediated by MSCs or DACCs (Ding and Pickard, 1993; Plieth, 1999; Plieth et al., 1999; White and Ridout, 1999; White, 2009). The wealth of potential ligands associated with VP generation (see ‘Presumptive significance of plasmodesmal connectivity for lateral VP dispersion’) renders the presence of ligand-activated channels on the sieve element plasma membrane highly plausible.
Location of the Ca2+-permeable channels in sieve elements
Early studies using BODIPY-DHP and antibodies localized voltage-dependent Ca2+-permeable channels to the sieve element (Volk and Franceschi, 2000). A more detailed approach using fluorochrome mixtures (Furch et al., 2009) and reaching a higher spatial resolution visualized Ca2+-permeable channels located in the plasma membrane and the endoplasmic reticulum (ER) stacks of sieve elements (Fig. 2B). Ca2+-permeable channels are unevenly localized to the sieve element plasma membrane. They are mostly aggregated in the vicinity of sieve plates and unilaterally branched plasmodesmata (pore–plasmodesm units; PPUs) towards the companion cells (Furch et al., 2009); hence, at the sieve element side facing the companion cell (Fig. 2B). A false-colour presentation of the ratio between BODIPY-DHP and RH-414 fluorescence confirmed preferential aggregation of Ca2+-permeable channels near sieve plates and PPU orifices (Fig. 2C). Furthermore, distribution of Ca2+-permeable channel clusters closely matched that of sieve element ER distribution in sieve elements (Fig. 2D), as was documented by double-label experiments (Fig. 2E).
Deployment of other channels and pumps involved in EPW propagation
Although this review does not focus on other channels and pumps involved in EPWs, we will pay some marginal attention to the few facts known. Whilst information on sieve element Cl– channels is entirely lacking, phloem-localized K+ channels of the AKT2/3 type were electrophysiologically characterized and linked to AP depolarization (Marten et al., 1999; Bauer et al., 2000; Lacombe et al., 2000; Deeken et al., 2002). Weak inward rectifying currents matching the features of AKT2/3 channels were recorded in sieve element protoplasts (Hafke et al., 2007, 2013). The increasing permeability of AKT2/3 channels at more alkaline pH values (Marten et al., 1999) and the extracellular alkalinization during transmission of SPs (Zimmermann et al., 2009) indicate that AKT2/3 channels are involved in membrane repolarization. As regards proton pumps, immunological approaches localized H+-ATPases to sieve elements and companion cells (Langhans et al., 2001).
Symplasmic organization of phloem strands
Ultrastructure and plasmodesmal connectivity of sieve elements
In sieve elements, cellular substructure is reduced to a plasma membrane envelope lined with a thin margin of gelatinous cytoplasm (mictoplasm) containing a limited number of organelles (e.g. van Bel, 2003). Originally, the mictoplasm was defined as the mixture of cytoplasmic contents with the sieve element fluid (Engleman, 1965). This layer has been re-defined as mictoplasm for practical reasons (van Bel, 2003): Ca2+ concentrations vary greatly in this space during EPWs (Furch et al., 2009).
The fact that the mictoplasmic layer is in open contact with the sap stream in the sieve element lumen is the consequence of tonoplast disintegration during sieve element ontogeny (Esau, 1969). Several organelles such as the nucleus, ribosomes, and Golgi apparatus are degraded during sieve element development (Behnke and Sjolund, 1990). The ER that may originate from the cortical ER (Hepler et al., 1990) survives the partial programmed cell death and is aggregated in regular stacks that are often oriented perpendicularly to the plasma membrane (Sjolund and Shih, 1983; Ehlers et al., 2000). The ER stacks are tethered to the plasma membrane and to each other by anchors of unknown nature (Ehlers et al., 2000) to prevent dragging by mass flow and resultant sieve pore occlusion. For the same reason, a special type of plastids of unknown function—considerably smaller than chloroplasts (Behnke and Sjolund, 1990)—are tethered to the plasma membrane (Ehlers et al., 2000).
Microscopically visible clusters of phloem-specific structural proteins are located at the margins of the sieve element (Behnke and Sjolund, 1990; Knoblauch and van Bel, 1998) or even in the sieve-tube lumen (Froelich et al., 2011; Knoblauch and Oparka, 2012). In addition, there is a wealth of soluble proteinaceous components in sieve elements (Barnes et al., 2004; Walz et al., 2004; Giavalisco et al., 2006; Aki et al., 2008; Furch et al., 2010; Dinant and Lucas, 2013). It has been excluded for a long time that a complete cytoskeleton exists in sieve elements (Parthasaraty and Pesacreta, 1980; Thorsch and Esau, 1981; Evert, 1990), although circumstantial structural (Chaffey and Barlow, 2002) and chemical (Kulikova and Puryaseva, 2002; Barnes et al., 2004; Walz et al., 2004; Giavalisco et al., 2006; Aki et al., 2008) evidence favoured the opposite view. Recent confocal laser scanning microscopic, immunological, and physiological studies have probably ended the dispute by identification of a complete, parietally located actin network in sieve elements (Hafke et al., 2013).
As inferred from dye coupling experiments, the sieve element precursor divides longitudinally and becomes transiently isolated from its neighbouring cells, which has been regarded as an instrument for developmental specialization (van Bel and van Rijen, 1994). The other daughter cell develops into 1–4 companion cells flanking each sieve element (Esau, 1969). Given its limited cellular equipment, a sieve element relies almost completely on its companion cell(s) for its survival, which makes communication between the two of paramount importance (van Bel 2003). Towards the end of the temporary symplasmic seclusion, so-called PPUs (van Bel and Kempers, 1997) arise that have the capacity to traffic a vast spectrum of substances including macromolecules between sieve element and companion cell(s) (Imlau et al., 1999; Lucas et al., 2001, 2009).
Plasmodesmata between companion cells and phloem parenchyma are sparse (Kempers et al., 1998), which seems to present a symplasmic bottleneck. These plasmodesmata have never been studied in detail, but may be of special nature, since their opening state is related to source–sink relationships (Patrick and Offler, 1996; Hafke et al., 2005). Moreover, the phloem-specific clostero- and luteoviruses are unable to pass this symplasmic border and, hence, are contained inside the sieve element–companion cell complexes (Stewart et al., 2013).
Presumptive significance of plasmodesmal connectivity for lateral AP dispersion
The electrical conductivity of the sieve element plasma membrane, the longevity of the sieve elements, and the high electrical conductance of sieve pores make sieve tubes ideal conduits for long-distance electrical signalling (van Bel and Ehlers, 2005). The restriction of longitudinal AP propagation to the sieve tubes indicates a high degree of electrical resistance in the plasmodesmal pathway from sieve elements to other cells (Fig. 3). The scarcity of plasmodesmata between companion cells and phloem parenchyma cells in transport phloem (Kempers et al., 1998), which are closed under source-limiting conditions, would fulfil the requirements for electrical insulation (Patrick and Offler, 1996; Hafke et al., 2005).
On the other hand, it should be borne in mind that electrical currents are expected to pass plasmodesmata with extremely low molecular exclusion limits or even move along the membranes crossing the cytoplasmic sleeve. Moreover, permanent and full electrical insulation of sieve element–companion cell complexes is unlikely, as inferred from symplasmic unloading of excess photoassimilates under sink-limiting conditions (Patrick and Offler, 1996), to fill axial storage compartments along the phloem pathway rapidly. ‘Electrical leakiness’ (Fig. 3A) is indicated by small depolarizations of phloem parenchyma cells coincident with the passage of EPWs (Rhodes et al., 1996). All in all, there is a good chance that the electrical insulation of sieve tubes is incomplete. Voltage-dependent Ca2+ channels in sieve elements would be the initiators of longitudinal AP propagation that is diverted by electrotonic leakage bringing about Ca2+ influx into vascular parenchyma cells.
The concept of functional current leakage is supported by events in the excitable plant Mimosa pudica, in which long distances are covered by APs owing to an insulating sclerenchyma sheath around the sieve element–companion cell complexes (Fleurat-Lessard and Roblin, 1982). This shield is interrupted in the pulvini, where numerous plasmodesmata provide ample symplasmic access to flexor parenchyma cells (Fleurat-Lessard and Bonnemain, 1978) with inherent facilitation of current leakage. The flexor cells react to Ca2+ influx by instantaneous loss of osmotic substances giving rise to leaf and leaflet bending (Fleurat-Lessard and Bonnemain, 1978). These phenomena may exemplify less prominent events in non-excitable plants with lower rates of current leakage and less eye-catching reactions by the flanking parenchyma cells. Altogether, it appears that incomplete insulation of sieve tubes is not a defect, but highly functional in lateral dispersion of Ca2+ waves and Ca2+-mediated information.
Presumptive significance of plasmodesmal connectivity for lateral VP dispersion
While the lateral events accompanying APs allow a straightforward assessment, there is more room for speculation regarding VPs. Disturbance of the hydraulic equilibrium in xylem vessels leads to water intake by the adjacent parenchyma cells which causes membrane depolarization due to increased turgor (Malone and Stankovic, 1991; Stahlberg and Cosgrove, 1992, 1997; Mancuso, 1999; Davies, 2006). Therefore, receptor potentials are probably triggered here by mechano-sensitive Ca2+-permeable channels (probably MSCs), but the mode of subsequent lateral electrical transmission to sieve tubes is a matter of debate.
As a first possibility (Fig. 3B), pressure-induced receptor potentials activate voltage-dependent Ca2+-permeable channels (perhaps DACCs) that generate EPW propagation towards the sieve tubes. The second and, at first sight, most likely option (Fig. 3C) is that the turgor of all vascular cells including the sieve tubes rises by intake of water after vessel damage, as argued for the mechanisms of cucurbit phloem exudation (Zimmermann et al., 2013). According to this scenario, VP propagation results from the action of mechano-sensitive channels which perceive local turgor changes in each vascular cell. This concept explains the attenuation of VPs with distance, provided that the relaxation in the vessels is increasingly dampened further away from the site of damage. Nevertheless, a few essential problems remain with this concept. Why is the VP generation not almost equally rapid along the vascular pathway, because pressure loss must propagate very quickly. In other words, why does it take so much longer for the VP to be expressed far away from the site of wounding, and why is the reaction to crushing so much more vigorous than to cutting, although the number of vessels damaged is approximately identical?
Therefore, it has been postulated as a third alternative (Fig. 3D) that MSC-mediated Ca2+ influx triggers cascades that produce chemical signals (Ricca, 1916; van Sambeek and Pickard, 1976; van Sambeek et al., 1976; Boari and Malone, 1993; Malone, 1996; Stahlberg and Cosgrove, 1997; Mancuso, 1999; Pyatygin et al., 2008). Oligosaccharides as well as the peptide systemin in solanacean species (Narvaez-Vasquez and Ryan, 2004) are potential messengers triggering VPs (Thain et al., 1995; Moyen and Johannes, 1996) after docking to ligand-activated Ca2+-permeable channels (Fig. 3D). The period to accumulate sufficient second messengers which may be correlated with the degree of relaxation would explain the increasing lag time between wounding and VP generation along the pathway.
In view of the co-occurrence of diverse Ca2+-permeable channels in plasma membranes (Kudla et al., 2010), combinations of the above scenarios are likely to occur. Irrespective of the mode of lateral EPW transmission, open plasmodesmata are compulsory (Fig. 3B, D; van Bel et al., 2011a), unless information is transferred by lateral pressure waves and plasmodesmal opening is unnecessary or even counter-productive.
Creation of Ca2+ hotspots in sieve elements
Formation and necessity of Ca2+ hotspots
In legumes, Ca2+ concentration changes in sieve elements were monitored in vivo by innate bioindicators. Their sieve elements contain so-called forisomes (Knoblauch et al., 2001), giant multimodular, striated protein bodies up to 100 μm in length (Schwan et al., 2009; Peters et al., 2010; Tuteja et al., 2010). The frequently forked forisome tips may reside in the mictoplasm (Furch et al., 2009). It is unclear, up to date if the modular ‘forisomette’ structure (Schwan et al., 2009; Tuteja et al., 2010) and the forked tips are fixation or manipulation artefacts. Upon Ca2+ elevation in sieve elements, forisomes disperse and reach up to six times their contracted volume (Knoblauch et al., 2003, 2012; Peters et al., 2006), so that the sieve element is blocked and mass flow stops (Thorpe et al., 2010; Knoblauch et al., 2012). After removal of Ca2+, probably by Ca2+ pumps (Kudla et al., 2010; Huda et al., 2013), forisomes recontract to their original size (Knoblauch et al., 2003; Furch et al., 2007, 2009).
During passage of a sufficiently effective EPW (Fig. 4A), forisomes disperse instantaneously and recontract after 5–20min (Fig. 4B–E), which is indicative of sudden Ca2+ influx and gradual Ca2+ removal (Furch et al., 2007, 2009). The reversible forisome reaction has been observed in vivo and in vitro (Knoblauch et al., 2001, 2003; Furch et al., 2007, 2009). Forisomes may block sieve pores in response to damage to prevent loss of the precious sieve tube sap and invasion by phytopathogens (van Bel, 2003).
The fact that Ca2+ channels act as relay stations between EPWs and Ca2+ influx into sieve elements has been demonstrated by Ca2+ channel blockers (Fig 4F–I). Membrane-impermeant La3+ and Gd3+ ions as well as the membrane-permeant blockers nifedipin and verapamil prevented the usual forisome dispersion in response to distant burning (Furch et al., 2009). Inhibitory effects of nifedipin and verapamil seem to confirm that Ca2+ channels are located on ER membranes (see Fig. 2D, E).
In Vicia faba, Ca2+ concentrations in sieve tube sap collected via cut aphid stylets and in sieve element mictoplasm were in the range between 50 nmol and 100 nmol (Furch et al., 2009). These concentrations were similar to those measured in the cytoplasm of other cells (Malho et al., 1998; Trewavas, 1999), but were different from values (in the micromolar to millimolar range) reported for sieve tube sap collected in other studies (Fromm and Brauer, 1994; Brauer et al., 1998). Concentrations in the low nanomolar range indicate a classic role for Ca2+ as a signalling ion (White and Broadley, 2003; Kudla et al., 2010) in sieve elements (Furch et al., 2009). Remote burning triggered prolonged EPWs along sieve tubes (Furch et al., 2007, 2009; Hafke et al., 2009) that coincided with a rise to 200–500 nmol Ca2+ in the mictoplasm and dispersion of the forisomes (Furch et al., 2009). However, these Ca2+ concentrations fail to meet by a long way the 50 μmol threshold needed for in vitro forisome dispersion (Knoblauch et al., 2001, 2005; Furch et al., 2009). This discrepancy suggests special conditions in the sieve elements needed for forisome dispersion in vivo (Hafke et al., 2009).
Ca2+ concentrations exceeding a few hundred nanomoles might only exist temporarily at the cytoplasmic mouth of the Ca2+ channels (Trewavas, 1999). Such high local Ca2+ concentrations have been detected in animal cells (Llinas et al., 1992, 1995), but have not been documented as yet for plant cells. Fluorescent reporters (i.e. Oregon Green-488-BAPTA-1; Furch et al., 2009) used for Ca2+ level determination probably dissipate steep Ca2+ microgradients (Bolsover and Silver, 1991; Malho et al., 1998; Demuro and Parker, 2006). Thus, the absence of forisome reactions in the presence of fluorescent Ca2+ buffers can be interpreted as indirect evidence in favour of high concentrated Ca2+ microdomains (Ca2+ hotspots) in sieve elements (Furch et al., 2009; Hafke et al., 2009).
A prerequisite for establishing Ca2+ hotspots is the local aggregation of Ca2+-permeable channels (White and Broadley, 2003). Punctuate patterns (Fig. 4B) indicate clusters of Ca2+-permeable channels in the vicinity of sieve plates and around PPUs (Fig. 2B, C; Furch et al., 2009). Voltage-dependent Ca2+-permeable channels were also localized to ER membranes in sieve elements (Fig. 2E; Furch et al., 2009), so that voltage changes in the plasma membrane (Furch et al., 2007, 2009) conducted by protein anchors between the plasma membrane and ER (Ehlers et al., 2000) might activate voltage-dependent channels at the ER stacks (Klüsener et al., 1995; Klüsener and Weiler, 1999; McAinsh and Pittman, 2009).
Ca2+ release from ER cisternae (Fig. 4J) implies that these are intracellular Ca2+ sequestration compartments (Buchen et al., 1983; Sjolund and Shih, 1983). It is uncertain to what extent second messengers such as the inositol phosphates IP3 (inositol trisphosphate; Gilroy et al., 1990) and IP6 (myo-inositol hexakisphosphate; Lemtiri-Chlieh et al., 2003), or cADPR (cyclic ADP-ribose; Lecki et al., 1998) play a role in Ca2+ release from the ER stacks in sieve elements. Inhibition of APs by the phospholipase C inhibitor neomycin—– phospholipase C is involved in the formation of IP3—in a variety of plant species (Krol et al., 2003, 2004) hints at the engagement of second messengers.
An elevated mictoplasmic Ca2+ level may boost its own concentration by Ca2+-stimulated Ca2+ efflux from ER stacks in analogy to Ca2+-induced Ca2+ release at the tonoplast (CICRs, or calcium-induced calcium release channels; Bewell et al., 1999; Sanders et al., 2002). Similarly, Ca2+ would trigger presumptive Ca2+-dependent Ca2+ channels on the ER membranes (Fig. 4F; Furch et al., 2009; Hafke et al., 2009). Ca2+ recruitment from internal stores is an established event during cold shocks (Knight et al., 1996; Gong et al., 1998; White and Broadley, 2003). Further evidence (Furch et al., 2009; Thorpe et al., 2010; van Bel, 2011a) also points to the ER as an important Ca2+ store which seems a major reason why ER stacks have been retained during sieve element evolution (Sjolund and Shih, 1983; van Bel, 2003).
All in all, Ca2+ hotspots are probably created where high densities of Ca2+-permeable channels in the plasma membrane and an abundance of ER stacks meet (Fig. 4J; Hafke et al., 2009). In line with putative Ca2+ accumulation at these sites, the reactivity of forisomes increases when their tips are located in the vicinity of Ca2+ hotspots in sieve elements (Furch et al., 2009). Further functional support for Ca2+ hotspots is provided by the fact that the forisome tips, being positioned between the ER stacks, are the only forisome parts that disperse as a reaction to weaker stimuli (Fig. 4J). The frequently perpendicular orientation of the ER stacks (Ehlers et al., 2000) facilitates insertion of the tips into a space (Furch et al., 2009), where Ca2+ levels may reach the threshold value needed for forisome dispersion. The interstices of the ER offer an undisturbed microenvironment for creation of Ca2+ hotspots (Furch et al., 2009; Hafke et al., 2009).
Correlation between the Ca2+ concentration in hotspots and forisome responses
As argued above, forisomes can be regarded as innate indicators for the Ca2+ thresholds and the approximate Ca2+ concentration in hotspots. APs seem to generate low-concentrated hotspots, since APs seldom lead to forisome responses (Fig. 4J) or, if they do so, lead to a slight wiggling of the forisome tails or a partial dispersion of the tips. Forisome dispersion coincident with prolonged EPW profiles indicates strong accumulation of Ca2+ at sieve element hotspots in response to VPs (Fig. 4J). Violent stimuli (burning, crushing) trigger APs and VPs in parallel that will collaborate in generating Ca2+ influx, the more so as Ca2+ potentiates it own hotspot concentration via Ca2+ liberation from ER stacks (Fig. 4J; Hafke et al., 2009). Ca2+ hotspots could also be meaningful for callose synthesis since Ca2+ concentrations required for this reaction greatly exceed those in the sieve tube sap (Hafke et al., 2009; Furch et al., 2009), at least in vitro (Colombani et al., 2004).
Involvement of the sieve element cytoskeleton in EPW propagation
It has been known for a long time that cold shocks induce transient blockage of sieve tubes (Pickard and Minchin, 1990), which has been related to Ca2+ channel reactivity (Thorpe et al., 2010). Cold shocks [>0.5 °C s–1 (Thorpe et al., 2010) or 4.2 °C in less than a second (Hafke et al., 2013)] induced sieve element depolarization followed by forisome dispersion (Fig. 5A). The depolarization was strongly reduced by the Ca2+ channel blocker La3+, and forisome dispersion also failed to occur. The apparent cold-triggered Ca2+ influx was originally ascribed to gating of mechano-sensitive Ca2+-permeable channels (Fig. 5A; Thorpe et al., 2010) due to a change of the tensile force exerted on the plasma membrane. Involvement of the cytoskeleton, however, was not excluded given the resemblance between cold-induced Ca2+ influx into the mictoplasm and other cell types (Knight et al., 1996; Plieth et al., 1999; White, 2009).
The latter has become more plausible after the recent discovery of a complete, dense actin network in sieve elements (Fig. 5E; Hafke et al., 2013). The actin disruptor latrunculin A (Lat A) has similar inhibitory effects on the cold-induced events (depolarization and forisome dispersion) in the presence or absence of La3+ (Fig. 5B; Hafke et al., 2013). Their equal impact indicates that LatA and La3+ target the same Ca2+ influx mechanism that is linked in some way to actin action. All in all, the presumptive interaction between Ca2+-permeable channels and actin (Hafke et al., 2013) predicts that the cytoskeleton plays a pivotal role in EPW propagation.
Interaction between Ca2+ channels and the cytoskeleton in sieve elements is further supported by an intimate connection between the plasma membrane and the actin meshwork as indicated by dense anti-actin immunochemical labelling of the face of the plasma membrane (Fig. 5C; Hafke et al., 2013). Forisomes probably must be kept in position for optimal sensing of Ca2+ changes in hotspots, although no compelling evidence for anchoring has been obtained thus far. The virtual absence of actin on dispersed forisomes (Fig. 5D; Hafke et al., 2013) seems to exclude that forisomes are linked to actin, unless actin filaments are torn apart during the fixation procedure due to forisome swelling. Other modes of linkage could be provided by protein filaments of unknown nature that anchor sieve element organelles to the plasma membrane (Fig. 5F; Ehlers et al., 2000) or tubulin. As expected, tubulin occurs in sieve elements (JBH, unpublished results) based on preliminary experiments using the tubulin disruptor oryzalin. Actin and tubulin may be coupled to different Ca2+ channels, since the activity of depolarization-activated (Mazars et al., 1997; Thion et al., 1998) and mechano-sensitive (Wang et al., 2004; Zhang et al., 2007) Ca2+-permeable channels was modulated by microtubules and microfilaments, respectively, in other cell types.
The interaction between cytoskeleton elements and Ca2+ channels and the inherent cytoskeleton involvement in shaping Ca2+ signatures and triggering intracellular signal cascades (Mazars et al., 1997; Trewavas and Malho, 1997; Drøbak et al., 2004; Davies and Stankovic, 2006) may be of paramount significance for EPW propagation. The question now arises as to how actin and Ca2+-permeable channels are linked (Fig. 5F). In general, cytoskeleton disruptors that destabilize either F-actin (Liu and Luan, 1998; Wang et al., 2004; Zhang et al., 2007) or microtubules (Thion et al., 1998) affect the action of ion channels. Protein complexes designated as ‘transducons’, which consist of an aggregate of receptors, Ca2+-permeable channels, bound calmodulin, protein kinases, and phosphatases, have been invoked to explain the intimate interaction between Ca2+ and the cytoskeleton (Trewavas and Malho, 1997) via members of the NETWORKED superfamily (Deeks et al., 2012). Transducons have been proposed to be tethered by integrins (Trewavas and Malho, 1997; Knepper et al., 2011) to the plasma membrane and cell wall.
Ca2+-induced sieve element occlusion mechanisms: a safety design?
Full sieve element occlusion achieved by forisomes had been a matter of debate (Peters et al., 2006) until in vitro experiments demonstrated that the swelling capacity was more than sufficient (Knoblauch et al., 2012). In intact V. faba plants, forisomes dispersed within seconds after EPW passage induced by burning and recontracted after 10–20min (Fig. 6A–C; Furch et al., 2007, 2009). Forisome dispersion turned out to be quicker than callose production (Furch et al., 2007, 2009). By the time that a forisome had recontracted, probably due to active Ca2+ removal (e.g. Huda et al., 2013), callose build-up reached its maximum followed by a slower degradation up to 3h (Fig. 6D; Furch et al., 2007, 2008, 2010). Both modes of occlusion are under the control of Ca2+ ions (Fig. 6E, F), the difference being that protein-mediated occlusion may have a lower Ca2+ threshold (50 μM; Furch et al., 2009; Hafke et al., 2009) than callose synthesis. In vitro callose synthesis required a concentration of 8mM Ca2+ (Colombani et al., 2004). Alternatively, the time lag of maximal callose deposition under the control of the Cal7 gene (Barratt et al., 2011; Xie et al., 2011) is due to the relative slowness of the complex de novo callose synthesis (Chen and Kim, 2009) with a Vmax of 45.5 nmol min–1 mg–1 (Li and Brown, 1993).
A dual sieve plate occlusion mechanism was also found in Cucurbita maxima (Furch et al., 2010). Rapid, apparent coagulation of the phloem proteins, PP1 and PP2, several centimetres away from the site of burning preceded callose deposition (Furch et al., 2010). As shown for various species, callose deposition reaches its maximum after 10–30min and is gradually degraded thereafter (Furch et al., 2008). Commensurate with the amount of callose deposited, PPUs reopen before the sieve pores do (Furch et al., 2007, 2008, 2010). Its occurrence in systematically distant families suggests that dual occlusion is widespread and functions to safeguard sieve tube contents. In this safety design, protein occlusion guarantees quick sieve plate sealing, which bridges the time until callose deposition is completed (van Bel et al., 2011a).
Although evidence in favour of a dual occlusion mechanisms is growing, numerous questions have to be addressed, in particular concerning the diversity of occlusion mechanisms. (i) It is unclear if the Ca2+ thresholds for protein reactivity and callose synthesis are different (Fig. 6E, F). There might be a vast spectrum of Ca2+ thresholds needed for diverse occlusion mechanisms (Furch et al., 2007, 2008, 2009, 2010), which in particular pertains to VPs which are positively related to the stimulus strength (Stahlberg and Cosgrove, 1997; Stahlberg et al., 2006). (ii) Most probably, not every protein clogging event in sieve tubes is Ca2+ dependent. Forisomes comprise SEO proteins (Pélissier et al., 2008), a widespread family among dicotyledons (Rüping et al., 2010; Anstead et al., 2012; Ernst et al., 2012; Jekat et al., 2012). SEO proteins are claimed to be Ca2+ binding in general (Ernst et al., 2012), although firm direct evidence seems to be lacking. Furthermore, PPs in cucurbit sieve tubes (Cronshaw and Sabnis, 1990; Dinant et al., 2003) do not belong to the SEO family (Ernst et al., 2012) and may react to (reactive) oxygen (species) or interact due to oxidation (Alosi et al., 1988). (iii) Since structural phloem-specific proteins are virtually absent in grasses (Eleftheriou, 1990), protein occlusion seems less important there (van Bel, 2003). However, emergence of protein plugs in gramineous sieve tubes indicates the presence of soluble proteins that are able to coagulate in response to injury (Will et al., 2009). (iv) The capacity to remove Ca2+ from sieve elements may be decisive for the reversibility of occlusion and achieved by a battery of Ca2+ efflux facilitators (Kudla et al., 2010; Huda et al., 2013). Their activities bear strongly on the mechanisms of Ca2+ homeostasis in sieve elements. Ca2+ efflux facilitators such as Ca2+ ATPases at the plasma membrane and the endomembranes, as well as Ca2+ exchangers (McAinsh and Pittman, 2009; Kudla et al., 2010) could modulate Ca2+ signatures and are responsible for cytoplasmic Ca2+ homeostasis. It has been speculated that soluble Ca2+-binding proteins fine-tune and shape Ca2+ transients during signalling (McAinsh and Pittman, 2009). Given the wealth of soluble proteins in sieve tube sap (e.g. Nakamura et al., 1993; Lin et al., 2009; Gaupels et al., 2012; Dinant and Lucas, 2013), this type of Ca2+ sequestration may be of paramount importance for Ca2+ buffering in the sieve element. Ca2+-binding proteins associated with the cytoskeleton could also act as modulators of Ca2+ signatures (Malho et al., 1998).
Relationships between Ca2+-mediated sieve element occlusion and pathogenic attacks
Apart from the involvement of Ca2+-permeable channels in the long-distance signalling of pathogenic infections and the implementation of defence mechanisms (e.g. Lecourieux et al., 2006; Cheval et al., 2013), Ca2+ channels are also locally and directly involved in putting up anti-pathogenic barriers. As reported below, penetration of aphid stylets and the presence of phytoplasmas elicit sieve tube occlusion mechanisms related to Ca2+ influx.
Aphid infestation
Sieve elements in Lupinus albus and V. faba occlude instantaneously by virtue of forisome dispersion in response to micropipette impalement (tip diameter 1 μm) due to Ca2+ influx (van Bel and van Rijen, 1994; Knoblauch and van Bel, 1998). After impalement, cell wall Ca2+ will diffuse into the sieve element via the wound edges created by the micropipette (Fig. 7A; Will and van Bel, 2006). Concomitantly, sieve element turgor is dissipated by the large micropipette volume, which may affect the gating of mechano-sensitive Ca2+-permeable channels (Fig. 7A). Remarkably, aphid stylets which have a similar tip diameter do not cause forisome dispersion (Walker and Medina-Ortega, 2012). Pressure loss into the stylet is minimal due to the minute volume and sealing of the wound edges by gel saliva (Miles, 1999; Tjallingii, 2006; Will et al., 2013) so that passive Ca2+ influx and activation of Ca2+-permeable channels are constrained (Fig. 7A; Will and van Bel, 2006).
Direct Ca2+-mediated interaction between sieve element reponses and pathogenic attacks. (A) In contrast to the strong Ca2+ influx following insertion of microelectrode tips, aphids are able to reduce Ca2+ influx in response to insertion of stylets with the same tip diameter. Aphids tightly seal the stylet penetration site by gel saliva to prevent Ca2+ influx. Moreover, closure of the stylet valve strongly reduces the loss of sieve element turgor pressure, keeping the mechano-sensitive Ca2+-permeable channels mainly inactivate (modified after Will T, van Bel AJE. 2006. Physical and chemical interactions between aphids and plants. Journal of Experimental Botany 57, 729–737; with permission of the Society for Experimental Biology.) (B–D) Observations of aphid behaviour using electrical penetration graph (EPG) recordings of sieve element-related aphid activities before, during, and after remote burning (B; Will T, Tjallingii WF, Thönnessen A, van Bel AJE. 2007. Molecular sabotage of plant defense by aphids. Proceedings of the National Academy of Sciences, USA104, 10536–10541 Copyright (2007) National Academy of Sciences, U.S.A.). Before burning, the waveform E2 (C) indicated ingestion of phloem sap. Several seconds after burning (marked by H), an abrupt change to waveform E1 (D) reveals that continuous secretion of watery saliva was observed. (E–I) Reaction of an isolated Vicia faba forisome (E; forisome condensed) to Ca2+ (F; forisome dispersed), EDTA (G; forisome condensed), Ca2+ again (H; forisome dispersed), and watery saliva concentrate (I; forisome condensed) (Will et al., 2007). (J–M) Forisome conformation associated with the presence of phytoplasmas (from Musetti R, Buxa SV, De Marco F, Loschi A, Polizzotto R, Kogel K-H, van Bel AJE. 2013. Phytoplasma-triggered Ca2+ influx is involved in sieve-tube blockage. Molecular Plant-Microbe Interactions 26, 379–386; with permission of the American Phytopathological Society). In control plants, the forisomes (f) are condensed in fluorescent (J) and light transmission (K) images obtained by confocal scanning microscopy, whereas the forisome is dispersed in phytoplasma-infected sieve elements (L, M). (N and O) Fluorescence images showing that the Ca2+concentration in control sieve elements (N) is much lower than in phytoplasma-infected sieve elements (O). (P and Q) Fluorescence images showing stronger callose deposits (blue) in infected (Q) as compared with control sieve elements (P).
When sieve element occlusion is triggered by remote burning, feeding aphids react within a few seconds (Will et al., 2007; Furch et al., 2010). Several aphid species switch from ingestion to secretion of watery saliva probably to counteract sieve tube occlusion by Ca2+ binding (Fig. 7B–D; Will et al., 2007, 2009). In vitro studies using forisomes (Will et al., 2007), biochemical techniques (Will et al., 2007), and proteomics (Carolan et al., 2009; Rao et al., 2013) confirmed that watery saliva contains Ca2+-binding proteins. In vitro, dispersion of Ca2+-treated forisomes was reversed by addition of the Ca2+ chelator EDTA or watery saliva concentrate from the aphid species Megoura viciae (Fig. 7E–I; Will et al., 2007). As a supplementary function, Ca2+-binding proteins may interfere with Ca2+-mediated defence and signalling mechanisms (Will and van Bel, 2008). Up-regulation of genes encoding calmodulin, calmodulin-like proteins, calcium-dependent protein kinases, and calcium-binding reticulin in response to infestation (Coppola et al., 2013) suggests a major role for Ca2+ in plant defence against aphids.
The fact that Ca2+-binding proteins have been identified in the phloem-feeding green rice leafhopper (Hattori et al., 2012) indicates that comparable strategies for suppression of plant defence may exist in diverse hemipteran families. Thus far, however, evidence in favour of in vivo suppression of Ca2+-induced sieve element occlusion by aphid saliva is lacking. On the contrary, forisome reversibility after leaf tip burning was found to be similar in distant sieve tubes with or without aphid stylet penetration in intact broadbean plants (Medina-Ortega and Walker, 2013).
Infection by phytoplasms
Phytoplasmas are frequently transmitted to plants by phloem-feeding leafhoppers and distributed via the sieve tubes (Christensen et al., 2005; McLean and Hogenhout, 2013) which become occluded in response to the infection (Braun and Sinclair, 1978; Kartte and Seemüller, 1991; Musetti and Favali, 1999). Phytoplasma-infected sieve tubes in V. faba contain consistently dispersed forisomes (Fig. 7J–M) hinting at Ca2+ levels appreciably higher than in healthy sieve tubes (Musetti et al., 2013). The Ca2+ concentration is indeed higher in infected sieve tubes (Fig. 7N, O). Moreover, the sieve tubes are sealed with thick deposits of callose (Fig. 7P, Q; Musetti et al., 2013). Undoubtedly, phytoplasmas induce Ca2+ influx with inherent consequences for forisome dispersion and callose synthesis. Thus far, it is unclear if sieve element occlusion is part of the plant's strategy against phytoplasma spread or if phytoplasmas induce and explore symplasmic isolation for undisturbed multiplication.
Physiological and genetic impact of EPWs
A diversity of physiological and genetic remote responses to EPWs have been reported, many of which are likely to be due to Ca2+ influx (Kudla et al., 2010). EPWs induce the expression of the proteinase inhibitor gene (pin2; Wildon et al., 1992; Pena-Cortes et al., 1995; Stankovic and Davies, 1997) and other genes (Davies, 2004) in distant parts of tomato plants. EPW propagation and gene expression are linked by the fact that Ca2+ influx and a consequent, transient increase in cytosolic Ca2+ is required for pin2 gene expression (Fisahn et al., 2004). Interestingly, the level of IP3, a second messenger potentially responsible for Ca2+ liberation from ER stacks (Gilroy et al., 1990; Krol et al., 2003, 2004), abruptly increases after EPW passage (Davies, 2004). Furthermore, touch-triggered EPWs evoke an arsenal of transcriptional downstream responses pertinent to 2.5% of the genes (Braam, 2005) including the enhanced expression of Ca2+-binding proteins (Lee et al., 2005). These data strongly suggests a relationship between EPWs, Ca2+ influx, and remote effects on gene expression.
A range of physiological responses to EPWs have been documented (Retivin et al., 1997; Fromm and Lautner, 2012). A Ca2+-controlled shutdown of proton pumps (Kinoshita et al., 1995; Hafke et al., 2013) during and after passage of a VP triggered by heating led to a transient decrease in the cytosolic pH from 7.0 to 6.4 and a concomitant increase of the apoplasmic pH from 4.5 to 5.2 (Grams et al., 2009). The lowered cytosolic pH, in turn, may bring about depressed CO2 uptake rates and reduced photosynthetic quantum yields (Koziolek et al., 2004; Lautner et al., 2005; Grams et al., 2009). In keeping with these observations, occasional sudden changes in osmotic potential of sieve tubes (Knoblauch et al., 2001) or pressure waves may cause VPs involved in the restoration of the source–sink balance. Remarkably, APs triggered by sudden flooding of drought-stressed roots produced the opposite effect: they induced an increase in stomatal conductance and photosynthesis (Grams et al., 2007) in the absence of appreciable pH changes.
Responses of distant cells to EPWs may depend on two major factors: the Ca2+ signatures in question and the equipment of the recipient cells. Ca2+ signatures (McAinsh and Pittman, 2009) will depend on the Ca2+-permeable channels involved, their cellular location, their Ca2+-mediated interaction, the available Ca2+ binding components, and the final Ca2+ compartmentation. There is a wealth of data on Ca2+ receptors and downstream signalling cascades (e.g. Dodd et al., 2010), and it seems that Ca2+ influx is of crucial importance for initiation of numerous cascades (Sanders et al., 2002; Kudla et al., 2010).
The diverse Ca2+ reactivity of various cell types has not yet been studied in detail, but there are obvious differences. Differential Ca2+ responsiveness of diverse cell types along the phloem pathway is exemplified by: sieve plate occlusion in sieve elements (Furch et al., 2007, 2009), production of NO in companion cells (Gaupels et al., 2008), systemin production in phloem parenchyma cells (Narvaez-Vazquez and Ryan, 2004), and massive water release in pulvinar flexor cells (Fleurat-Lessard and Bonnemain, 1978). It will be fascinating to explore further the impact of EPWs on gene expression, metabolism, and physiology of distant conductive elements and adjoining cells.
Speculations on whole-plant effects of EPW-modulated Ca2+ waves
Symplasmic organization of phloem strands changes in response to Ca2+ fluxes
Ca2+ influx during APs mostly is insufficient to induce forisome dispersion and sieve element occlusion (Fig. 4J). After passage of EPWs of sufficient strength, namely VPs or, in particular, a combination of VPs and APs (Fig. 4J), Ca2+ levels exceed an activation threshold. The resultant occlusion of the intercellular corridors may impose a transient symplasmic reorganization of the sieve tube tracks (Fig. 8). Apart from the proven occlusion of sieve plates and PPUs (Figs. 4,6), elevated Ca2+ levels in the adjoining parenchyma cells may induce the deposition of callose collars around their plasmodesmata, as demonstrated for several tissues (e.g. Tucker, 1990; Kauss and Jeblick, 1991; Radford et al., 1998; Holdaway-Clarke et al., 2000; Sivaguru et al., 2000, 2005; Michard et al., 2011). Formation of callose deposits blocking photoassimilate loading by sieve tubes in response to APs (Fromm et al., 2013) is in agreement with this concept.
Presumptive Ca2+ influx events during a mixed EPW after severe damage conferring symplasmic reorganization. When AP and VP waves are rolling along the sieve tubes, the quicker AP is dispersed into the adjacent cells at the AP forefront. The VP that results from local depolarizations propagating towards the sieve elements propagates at slower rates. When the Ca2+ concentrations in sieve elements surpass a threshold, sieve pores and PPUs are occluded (crosses) transiently so that the sieve elements become stagnant and symplasmically isolated. Simultaneously, elevated Ca2+ concentrations in vascular cells may cause plasmodesmal closure (crosses with question marks) so that cells also gain a temporary autonomy. Voltage-dependent and mechano-sensitive Ca2+ permeable channels in violet and lilac, respectively.
Without the usual interaction with their neighbours, occlusion of symplasmic contacts would render vascular cells temporarily autonomous units (Figs. 8,9). Under these conditions, vascular cells may be able to switch to other cascades implementing more discrete metabolic or genetic programmes. The effects of (transient) plasmodesmal closure on cell autonomy were demonstrated for the differentiation of the stomatal apparatus (Palevitz and Hepler, 1985), the divergent development of the sieve element and companion cell (van Bel and van Rijen, 1994), the formation of symplasmic domains (Ehlers et al., 1999), the synchronization of metabolic activity (Ehlers and Kollmann, 2000), and the explosive elongation of cotton hair cells (Ruan et al., 2001). As soon as the lifelines (PPUs) between companion cells and sieve elements have been restored, Ca2+-induced products can be released into the sieve elements and translocated to target cells when the sieve pores become re-opened (van Bel et al., 2011a).
Hypothetical wave-like behaviour of systemic signalling induced by EPWs. There may be a distinct correlation between systemic phloem signals associated with APs (mostly Ca2+ influx below the occlusion threshold) or with VPs (mostly Ca2+ influx above the occlusion threshold). In the absence of sieve element occlusion (left panel), the first wave of signalling (I) comprises free Ca2+ ions and Ca2+-conjugated sieve element proteins arriving in the target cells. The second wave of signalling (II, left) includes constitutive and readily available low molecular weight agents released from the vascular cells. The last wave of signalling (III, left) may include macromolecules (proteins, RNAs) the synthesis of which in the vascular cells requires a long period. With sieve element and/or plasmodesmal occlusion (right panel, II; see also Fig. 8), the composition of the signals in waves III and IV (right panel) might differ from those in the left panel given the transient symplasmic isolation of cells in the sieve tube track (II).
Ca2+-triggered systemic signalling occurs in partly overlapping waves
Lateral transfer of EPWs, either focused in the pulvini (Fleurat-Lessard and Bonnemain, 1978) or distributed along the entire pathway (Rhodes et al., 1996), may reflect a fundamental difference between EPWs in animals and plants. Instead of the minor ion displacements occurring in animals, gating of ion channels causes massive ion displacement in plants (Pyatygin et al., 2008). Apart from the regulation of Ca2+ influx, ion displacement in plants may strongly contribute to ion homeostasis (e.g. Mummert and Gradmann, 1991; Trebacz et al., 1994; Zimmermann and Felle, 2009).
Dissemination of electrical signalling implies that both cells along the phloem pathway and those at the termini of the phloem track are targets for EPWs. The multitude of potential combinations of Ca2+ influx and its differential effects on diverse cell types potentiate the complexity of the responses and provide an endless wealth of possibilities (Kudla et al., 2010; Dempsey and Klessig, 2012) exemplified by the ‘myriad plant responses’ to herbivores (Walling, 2000).
We explore the possibility—as advanced before in a less elaborate way (van Bel and Ehlers, 2005)—that phloem-borne signalling passes through partly overlapping waves which are distinct in time scale, site of origin, and nature (Fig. 9). At the forefront of EPWs, Ca2+ ions are released into sieve elements which may readily attach to constitutive Ca2+-binding proteins in the sieve tube sap such as Ca2+-dependent protein kinases (Nakamura et al., 1993; Yoo et al., 2002; Gaupels et al., 2012). Thus, the first wave of signals (time scale: seconds to minutes to arrive in target cells) may include free Ca2+ ions accompanied by Ca2+-activated or Ca2+-binding proteins. As a result, Ca2+ signatures induce proactive responses to imminent changes. The signatures will depend on the stimulus; that is, disparate signatures are obtained from diverse Ca2+-permeable channels which are functionally linked with different cytoskeleton components (Mazars et al., 1997; Thion et al., 1998; Wang et al., 2004; Zhang et al., 2007).
A second wave of signals (time scale: minutes to hours) may comprise compounds from the vascular parenchyma that are readily manufactured under the control of Ca2+ influx. If the EPW is accompanied by symplasmic reorganization, the longer residence time could make the stagnant contents of sieve elements into reaction vessels for Ca2+ binding to constitutive sieve element proteins, and vascular cells may follow alternative signalling cascades as argued above. Thus, without sieve element occlusion, the compounds released into the sieve tubes for further translocation may differ from those released after relief of symplasmic re-organization (Fig. 9). During this second stage, various parallel cascades may be initiated by Ca2+ influx. For instance, calmodulin-like and calmodulin (McCormack and Braam, 2003; Lee et al., 2005; McCormack et al., 2005), as well as other specific Ca2+-binding proteins (White and Broadley, 2003; Kudla et al., 2010) are attached to the cytoskeleton (Malho et al., 1998). In this way, information conferred by Ca2+ signatures is decoded and transformed into protein–protein interactions, resulting in Ca2+-dependent phosphorylation cascades like transcriptional responses that lead to downstream reactions (Luan et al., 2002; Sanders et al., 2002; Kudla et al., 2010).
Whether Ca2+ is directly related to the synthesis of jasmonic acid (Fisahn et al., 2004) and/or salicylic acid is uncertain, but there seems little doubt that Ca2+ ions are engaged in the action of jasmonic acid (Munemasa et al., 2011) and salicylic acid (Du et al., 2009; Boursiac et al., 2010). In addition, cytosolic Ca2+ elevation is linked to downstream nitric oxide production, as shown for companion cells (Gaupels et al., 2008) via the intervention of calmodulin-(like) proteins (Ma et al., 2008).
The third wave (time scale: hours) would encompass long-term implementation of Ca2+ effects exemplified by the production of various types of RNA (Kehr and Buhtz, 2013), proteins (Lin et al., 2009; Dinant and Lucas, 2013), and even lipidic substances (Guelette et al., 2012) present in the sieve tube sap. For the impact of Ca2+ signals on the production of macromolecules, the reader is referred to an excellent review (Kudla et al., 2010), but a few examples are given here. Ca2+ signals are converted into transcriptional responses for a fair number of genes (Lee et al., 2005; Kaplan et al., 2006) which may comprise ~3% of the protein-coding genes in Arabidopsis (Kudla et al., 2010). Many of these expression responses depend on Ca2+ regulation of the transcription factors (e.g. Finkler et al., 2007). As an interesting note in the present context, one of these transcription factors interacts with the promoter of AtEDS1, a regulator of salicylic acid synthesis (Du et al., 2009).
Macromolecules produced in the vascular cells and released into the sieve tube sap via PPUs (Lucas et al., 2001; Chen and Kim, 2006; Lough and Lucas, 2006; Ding and Itaya, 2007; Lin et al., 2009) might find their way to target cells by molecular tagging (zip codes) so that compounds required for local and remote use can be distinguished (van Bel et al., 2011b). In this way, macromolecules are recognized to remain within the sieve element into which they had been released or move either to companion cells along the pathway (Fisher et al., 1992; Golecki et al., 1999) or to sink cells. Interactions on the interface between ER stacks and the sieve element cytoskeleton may play a crucial part in the distribution of macromolecules inside the sieve element and delivery of macromolecules into the sieve tube sap. Presumably, some of the macromolecules are back-trafficked into companion cells by the aid of non-cell autonomous agents (Schulz, 1999; Itaya et al., 2000; Lucas et al., 2009). This ‘molecular hopping’ (van Bel et al., 2011b) may provide a complex basis for amplification or attenuation of systemic signals. Macromolecules enter sink cells via permanently widened plasmodesmata (Fisher and Cash-Clarke, 2000), each of which may demand specific entrance codes (Foster et al., 2002).
Concluding remarks
This review is a plea for further research on the link between EPWs and chemical systemic signalling. It appears to be worth investigating if and to what extent EPWs provide a common basis for the rapid distribution of Ca2+ signals. A limited number of studies demonstrate the immense and remote effects of EPWs on the genetics and physiology of plants. There may be a few prime targets for investigation.
Which Ca2+-permeable channels are involved in the propagation of EPWs and the processing of electrical information in vascular cells?
Is there an impact of a temporary symplasmic organization on the production of signalling substances?
Do the Ca2+ signatures and the resultant cascades depend on the nature of electrical signalling; for example, is there a difference between Ca2+ signatures induced by VPs or APs?
Do the Ca2+ signatures and the resultant cascades depend on the receptor or target cell types; that is, are there cell-specific responses to identical Ca2+ signatures?
What is the relationship between Ca2+ influx and the production of various mobile signals along the pathway such as phytohormones, reactive oxygen species, lipidic substances, proteins, and RNA species?



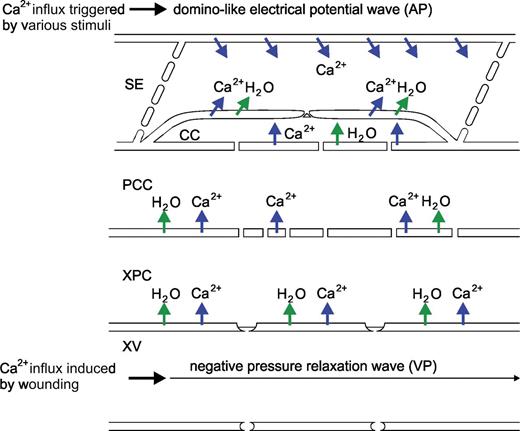
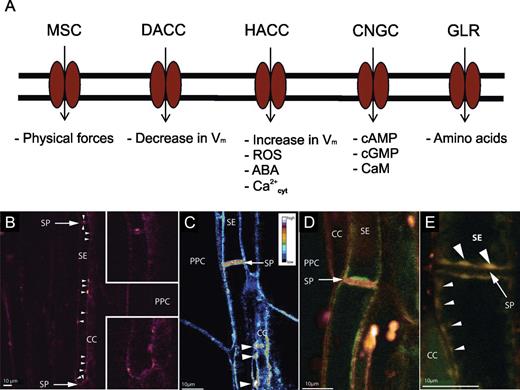
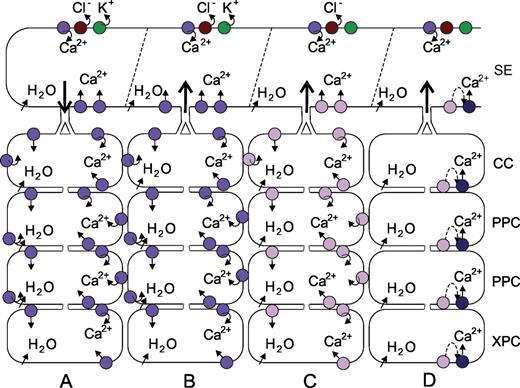
![Use of forisomes as indicators of Ca2+ concentration thresholds and the formation of Ca2+ hotspots in legume sieve tubes. (A) Microelectrode-recorded passage of an EPW triggered by remote burning (from Furch ACU, Hafke JB, Schulz A, van Bel AJE. 2007. Ca2+-mediated remote control of reversible sieve tube occlusion in Vicia faba. Journal of Experimental Botany 58, 2827–2838 with permission of the Society for Experimental Biology) in a sieve element. The arrows (B–E) indicate the time points at which confocal laser-scanning images of forisomes (asterisk) in the sieve element (SE) were taken. Condensed forisomes are visible (B and E), but dispersed forisomes are not (C and D) due to changed optical properties. The whitish spots are autofluorescent chloroplasts. Companion cells (CCs) and sieve plates are marked with arrowheads. (F–I) The effect of Ca2+ channel inhibitors on forisome dispersion (Furch et al., 2009) in response to remote burning (see B, C as controls). (F and G) Absence of forisome dispersion (asterisk) in the presence of La3+. Black arrowsheads point to electrode tips. (H and I) Absence of forisome dispersion (asterisk) in the presence of nifedipine. (J) The creation of Ca2+ hotspots. Hypothetical deployment of Ca2+-permeable channels, the ‘EPW strength’, and the influx of Ca2+ ions (strongly modified after Hafke JB, Furch ACU, Fricker MD, van Bel AJE. 2009. Forisome dispersion in Vicia faba is triggered by Ca2+ hotspots created by concerted action of diverse Ca2+ channels in sieve elements. Plant Signaling and Behavior 4, 968–972). This model assumes that a graded stimulus–response correlation depends on the increasing engagement of diverse Ca2+-permeable channels with increasing stimulus strength. The latter results in cumulative Ca2+ release into the unstirred mictoplasm. The impact of the stimulus increasing from a to d is depicted by profile symbols representing the contribution of APs and VPs to the EPW and the putative Ca2+ influx. The grey tones demonstrate Ca2+ concentrations effected by various stimuli in the vicinity of ER–plasma membrane conglomerates which are connected by nano-anchors (see Fig. 5F). (a) Small AP-like depolarizations induce minute release of Ca2+ ([Ca2+]mict stippled arrow) via voltage-dependent Ca2+ channels (green symbols) into the mictoplasm. These may affect forisome attachment given the slight forisome movements, but are insufficient to bring about conformational changes of the forisome. (b) Larger AP- and VP-associated depolarizations enhance the micoplasmic Ca2+ level [Ca2+]mict to such an extent that forisomes move and/or swell. The voltage drops along the sieve element plasma membrane may be propagated via the minute protein anchors to the ER, where voltage-dependent Ca2+ channels are activated. The latter channels (green symbols) in the ER are needed to sustain the rise in Ca2+. Alternatively, or additionally, Ca2+-dependent Ca2+ channels may be activated to initiate Ca2+ release (CICR channels, yellow symbols) from the ER as reported for storage vacuoles (Bewell et al., 1999). This implies that Ca2+ ions are not released from ER cisternae without a preceding plasma membrane trigger. (c) Since cutting close to the site of observation causes a massive loss of turgor, a variation potential most probably overlaid with an AP transient induces Ca2+ release to an extent that causes full forisome dispersion. (d) A steep AP followed by a massive VP induces not only forisome dispersion, but also callose deposition. It is unclear if the VP is initiated by suppression of the plasma membrane proton pump activity leading to activation of voltage-gated channels or by direct influx via MSCs (red symbols) supplemented by Ca2+-activated Ca2+-permeable channels (yellow symbols). To elevate Ca2+ concentrations ([Ca2+]mict, thick arrow) further to the level required for callose synthesis, the Ca2+ signal may be amplified by involvement of signal molecules such as IP3. High-affinity IP3-binding sites on the ER suggest the presence of IP3-gated Ca2+ release channels (blue symbols). An increase in cellular IP3 levels has been reported in response to osmotic shocks (Munnik and Vermeer, 2010) and burning stimuli (Davies, 2004).](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/jxb/65/7/10.1093_jxb_ert425/3/m_exbotj_ert425_f0004.jpeg?Expires=1716430688&Signature=NYwA8Zue9HngOba3Gk-XG7b0RV6qmrZ0g9jMr3F2GAuPb~GlD-6BevCmMg3OADvYl-3nYlFeqQ6N-5qjG3s1OrHpZlaPl9GgvyPQ9D3eFYYAmN7Jgq37BFIEU4e0q32jZwmCf9JxdRr5b0Uh5ONpp6T8UnGsKQWxvghwE7NQYew-lPsvBcRgRrDRIC0bCGk~Du0mgRY-FA-m7bAX0rygRbcmAvZ-dUJmibYNzwZ9btbh1d~cFAT~jNx2uI2aiEZRILmKTb7WdiBO2eZZmnrWptH2qmXuAChR1Q-nw-Kc7rbXCTZJDb3DEo-q3PQhMeuYe7VtinBCROKgqSfCZQl6Ew__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
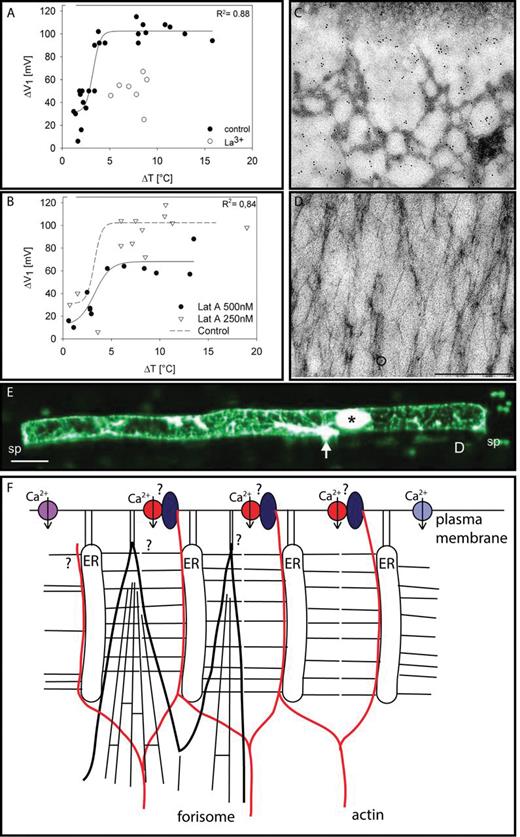
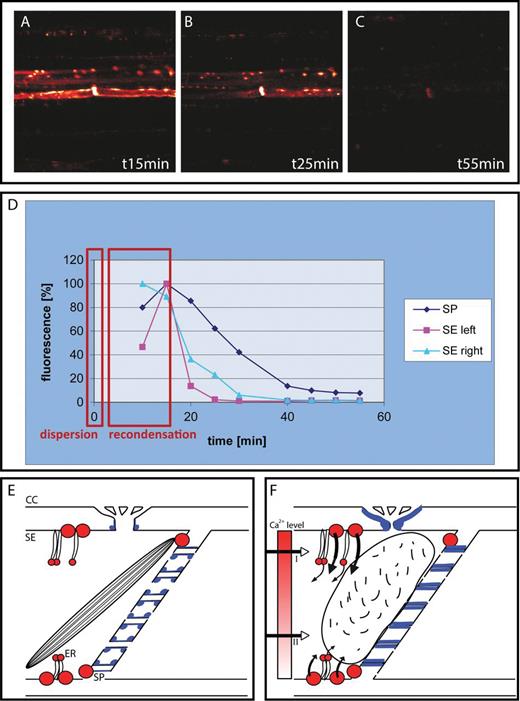
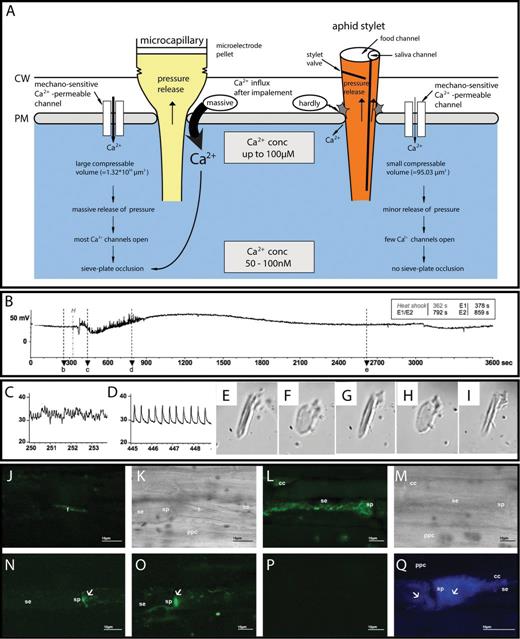
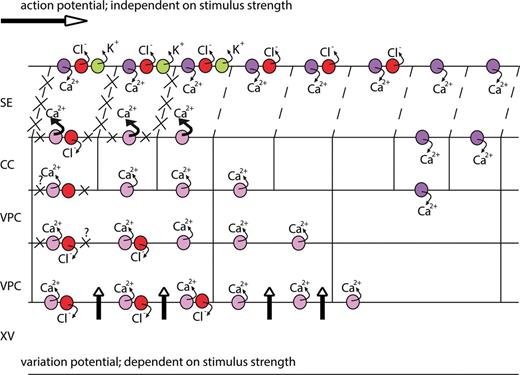
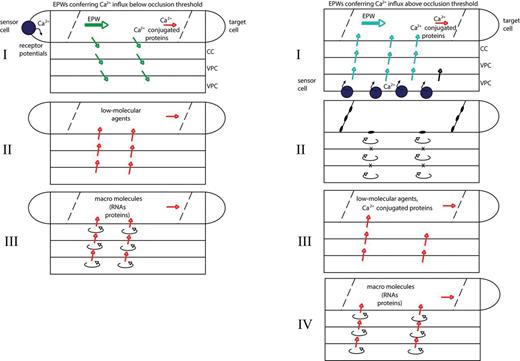

Comments